- Submissions

Full Text
Research in Pediatrics & Neonatology
Clinical Cases of Newborns with Severe Respiratory Failure
Bondar VA, Pushkov AA, Basargina MA, Zhanin IS, Davydova IV And Savostyanov KV*
National Medical Research Center for Children’s Health Federal state autonomous institution of the Russian Federation Ministry of Health, Russia
*Corresponding author: Savostyanov KV, National Medical Research Center for Children’s Health Federal state autonomous institution of the Russian Federation Ministry of Health, Russia
Submission: December 12 , 2022; Published: December 16, 2022

ISSN: 2577-9200 Volume7 Issue2
Abstract
In this case-report we describe clinical observations of two newborns with similar clinical and medical history data with severe respiratory failure at birth. One child had high genetic risk of bronchopulmonary dysplasia development (presence of rs45488997 in the CTGF gene), and the other had low risk (presence of rs12489516 in the CPA3 gene).
Keywords: BPD; Genetic predisposition; Children
Introduction
Bronchopulmonary dysplasia (BPD) is a multifactorial disease, that develops due to the interaction of prenatal and postnatal factors leading to impaired development of the lower respiratory tract and pulmonary vessels, as well as the formation of chronic lung pathology associated with respiratory failure [1]. Based on a molecular genetic study of 100 exomes of Russian children with BPD, we revealed genetic polymorphisms that modify the risk of BPDdevelopment in Russian children, that combined with clinical features allowed us to develop the algorithm for risk prediction of the new BPD form development [2].
Case Report: 1
Child N. was born from 25-year-old woman. The gestation course: 1 trimester-threatening miscarriage, retro chorial hematoma; 2 trimester-increased titer of anti-erythrocyte antibodies by 25th week, fetal anemia. The delivery course: operative delivery at 26th week of gestation. Child’s weight at birth-660g, length at birth-34cm. APGAR score 1/3 points.
The child’s condition after birth was extremely severe due to respiratory failure and neurological symptoms. Tracheal intubation and endotracheal adrenaline injections were performed in the delivery room. Exogenous surfactant drug was administrated to prevent newborn respiratory distress syndrome (NRDS) at the 14th minute of life. After stabilization of his condition, child N. was transferred to the neonatal intensive care unit and ventilation was continued. The need in strict ventilation parameters and high oxygen concentration remained during a month of being in the intensive care unit, thus, nitric oxide insufflation was performed to relieve pulmonary hypertension.
Buccal epithelium smear was taken from the patient for further molecular genetic analysis. DNA was isolated from buccal epithelial cells via the phenol-chloroform extraction method. Genomic library was prepared and enriched with a custom target panel (KAPA, Rosche USA) for massive parallel sequencing. Sequencing was performed on the Illumina Next Seq 550DX Instrument (Illumina, USA). Genetic variant rs45488997 was revealed in the CTGF gene according to the results of molecular genetic study, it is associated with the development of BPD in newborns (Figure1A).
Child was on a ventilator for 43 days of life due to severe respiratory insufficiency against the background of NRDS, respiratory support by the BIPAP method was carried out for 13 days, the supply of moistened oxygen through a facial mask for 6 days. From 62 day of life the child has been breathing independently without additional oxygenation.
Chest CT showed the inequality of lung tissue aeration with areas of subpleural emphysematous swelling of the lower parts of both lungs (Figure1B). There were no focal and infiltrative changes. The vascular pattern was accentuated and deformed due to transpulmonary cords. The walls of the bronchi are thickened, the lumen is free, not deformed. No fluid was detected in the pleural cavities and in the pericardial cavity.
Figure 1: Child N. had rs45488997 variant in the CTGF gene; (B): chest CT scan.
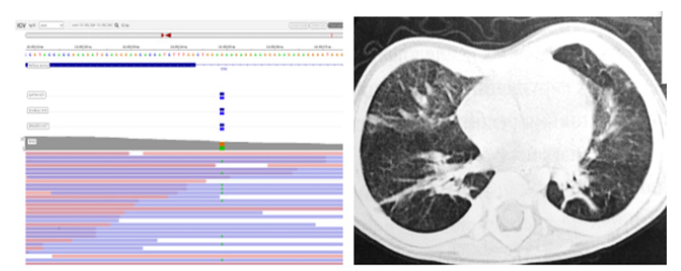
The patient was discharged home at the age of 2 months and 20 days with diagnosis of severe BPD, extreme immaturity (26th weeks), intraventricular hemorrhage, retinopathy of prematurity, moderate protein-energy malnutrition.
Previously developed scale was used to predict the risk of BPD developing. It includes 7 parameters: gestational age at birth, body weight, body length, APGAR score at 1 minute, APGAR score at 5 minutes, the presence of rs12489516 (CPA3) and the presence of rs45488997 (CTGF) (Table 1). Scores from 0 to 3 points correspond to low risk of developing a new form of BPD, from 4 to 7 points-high risk.
Table 1: Scale for predicting BPD development in newborns.
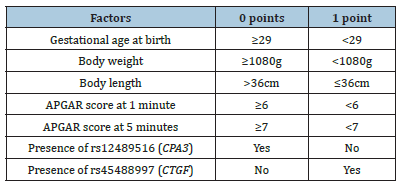
According to the described scale, patient N. had a high risk of developing BPD, estimated at 7 points.
Case Report: 2
Child S. was born from 22-year-old woman. The gestation course: 1 trimester - colpitis; 2 trimester - the threat of termination at 22nd weeks, moderate oligohydramnios. The delivery course: operative delivery at 31st week of gestation. Child’s weight at birth- 990g, length at birth-36cm. The APGAR score is 6/7 points.
The child’s condition after birth was severe due to respiratory failure. CPAP therapy was initiated in the delivery room, surfactant drug was administrated by the end of the 2nd hour of life. On the 6th day of life, considering the positive dynamics, CPAP respiratory therapy was discontinued, and the child started to supply moistened oxygen through nasal cannulas. The total duration of oxygen dependence was 15 days.
Cytomegalovirus infection was suspected due to cytolysis syndrome. PCR test showed a high titer of cytomegalovirus in saliva and blood, therefore specific therapy was initiated.
Buccal epithelium smear was taken from the patient for further molecular genetic analysis. The method of genetic analysis was similar to one described in the first clinical case. The analysis revealed genetic variant rs12489516 in the CPA3 gene associated with a reduced risk of BPD development in Russian newborns.
Ophthalmologist revealed retinopathy of prematurity, 2nd grade, active form.
Patient S. did not require additional oxygenation by 16th day of life and did not develop BPD. She was discharged home at the age of 2 months and 22 days with diagnosis of congenital cytomegalovirus infection, extreme immaturity (31st weeks), retinopathy of prematurity, severe protein-energy malnutrition. The BPD development risk according to the scale was 2 points.
Conclusion
Comprehensive diagnostic approach based on the analysis of clinical and anamnestic data, results of instrumental diagnostics, revealed molecular genetic variants makes it possible to effectively predict the new BPD form development in newborns. Despite comparable mass-growth rates and low gestational age at birth in both clinical cases, first child developed BPD, second one did not. Genetic testing in the early postnatal period reveals the presence of risk factors for the BPD development and allows us to adjust preventive and therapeutic approaches.
References
- Yu KH, Li J, Snyder M, Shaw GM, O'Brodovich HM (2016) The genetic predisposition to bronchopulmonary dysplasia. Curr Opin Pediatr 28(8): 318-323.
- Bondar VA, Davydova IV, Basargina MA, Fisenko AP, Pushkov AA, Zhanin IS, Borisov IV, Savostyanov KV (2022) The role of genetic predictors in the preclinical diagnosis of bronchopulmonary dysplasia. Kremlin Medicine 1: 4-9.
© 2022 Savostyanov KV. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






