- Submissions

Full Text
Research in Pediatrics & Neonatology
Mowat-Wilson Syndrome with Nonsense Mutation of ZEB2 Gene: Case Report and Review of the Literature
Lun Chin Lin1, Wan Hsin Wen1,2, Ni Chung Lee3,4, En-Ting Wu3, Kai Chi Chang3,5, Peir Taur Chen1* and Leticia B Sy1
1Department of Pediatrics, Cardinal Tien Hospital, Taiwan
2School of Medicine, College of Medicine, Fu-Jen Catholic University, Taiwan
3Department of Pediatrics, National Taiwan University College of Medicine and Hospital, Taiwan
4Department of Medical Genetics, National Taiwan University Hospital, Taiwan
5Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taiwan
*Corresponding author: Peir Taur Chen, Department of Pediatrics, Cardinal Tien Hospital, New Taipei City, Taiwan
Submission: July 23, 2022; Published: September 26, 2022

ISSN: 2577-9200 Volume6 Issue5
Introduction
Mowat-Wilson syndrome (MWS) is characterized by distinctive facial appearance in association with multiple congenital anomalies [1-5]. MWS is caused by heterozygous mutations or deletions in the zinc finger E-box-binding homeobox 2 gene, ZEB2 (2q22.3) [5,6]. To date, over 100 deletions/mutations have been reported in patients with a typical phenotype [6]. Prevalence is estimated at 1/50,000 to 1/70,000 live births [5]. To our knowledge, this is the first case of MWS in Taiwan fond to be in association with tracheal stenosis without the presence of pulmonary artery sling and which have not been reported in the previous case reviews.
Case Report
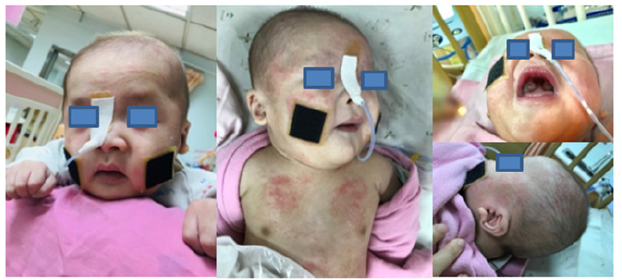
The case is a term male neonate with normal birth weight 2955g born to a healthy mother after an uncomplicated pregnancy. Thirty hours after delivery, he suffered from severe abdominal distention and minimal meconium passage. The diagnosis was later confirmed as Hirschsprung’s disease (HSCR) with transitional zone at the sigmoid-descending colon junction by rectal mucosal suction biopsy showing hypertrophied nerve fibers without calretininpositive nerve fibers. The neonate also showed dysmorphic facial features as described in the attached figure legend. Brain sonography revealed dysgenesis of corpus callosum, which was later confirmed by brain MRI. Bronchoscopy was indicated for difficult intubation procedures and tracheal stenosis with complete tracheal rings was found. Chest computed tomography also revealed mild segmental stenosis at the middle trachea without remarkable cardiovascular anomalies. The baby had clinical seizures with focal epileptic discharges in electroencephalography since around 3 months of age, and he remained seizure-free under anti-epileptic drug monotherapy. Later, genetic survey by Next-Generation Sequencing proved that there was a heterozygous NM_014795: c.2083C>T (p.Arg695Ter) variant in ZEB2 gene. According to ACMG interpretation criteria, this variant is classified as pathogenic (PVS1, PS1, PM1, PM2, PP4) (Figure 1).
Figure 1:It shows some distinctive facial features found in MWS: square-shaped face with high forehead, frontal bossing, hypertelorism, deep set but large eyes, broad nasal bridge, saddle nose with prominent rounded nasal tip and columella, open mouth with M-shaped upper lip, and uplifted earlobes.
Discussion
Observation of syndromic HSCR was the initial clue for the final diagnosis of MWS in this case. About half of the cases with MWS (44%-57%) were associated with HSCR according to the literature reviews [5,6]. However, HSCR is not present in every infant with MWS and therefore is not a required diagnostic criterion.
Facial dysmorphism may become evident during childhood. Characteristic features include uplifted earlobes with a central depression and large, medially sparse and flaring eyebrows [1,6]. Other non-specific facial features include square-shaped face with high forehead, epicanthal folds, hypertelorism, telecanthus, large deep-set eyes, strabismus, broad nasal bridge, rounded nasal tip, prominent columella, open mouth with M-shaped upper lip, and prominent but narrow and triangular pointed chin with excess nuchal skin and puffy anterior neck [1,6]. Our patient also showed characteristic uplifted earlobes in addition to some of other nonspecific features during infancy, but medially flaring eyebrows were not prominent.
Congenital heart defects and urogenital anomalies were noted in about half of the MWS cases, 52% and 51% respectively [6]. Other reported anomalies include callosal hypoplasia or agenesis (43%), pyloric stenosis (4.7%), structural eye anomalies (4.1%), cleft palate (2.9%), pulmonary sling with/without tracheal stenosis/ hypoplasia (2.9%) [6]. Interestingly, in our case, we found isolated tracheal stenosis without pulmonary sling which is a distinctive finding compared to previous MWS case reviews. Patients usually have moderate to severe intellectual disability with epilepsy [4]. Speech is absent or limited to a few words, with onset at around 5-6 years [6]. Epilepsy has a prevalence of 70-75% and has an agedependent electroclinical pattern [4]. All types of seizures have been reported. Resistance to antiepileptic drugs was reported in about one-fourth of patients with MWS1,3 but in our case, seizure was well-controlled with anti-epileptic drug monotherapy.
Genetic testing plays an important role in the diagnosis of MWS. Several types of mutations were found including large deletions, frameshift mutations (small deletions/insertions/indels), nonsense mutations, missense mutations, splice site mutations. Among them, the most frequently identified types of mutations were frameshift mutations (46%) and nonsense mutations (37.9%) [2]. In our case we found nonsense mutation of ZEB2 gene on exon 8 leading to nucleotide change (c.2083C>T) with a stop codon (p.Arg695*) and thus causing protein truncation.
In conclusion, MWS is a rare genetic syndrome with a distinctive phenotype caused by mutations in ZEB [2]. Interestingly, in our case, we found isolated tracheal stenosis without pulmonary artery sling which is a distinctive finding compared to the previous MWS case reviews. Better understanding of this genotype-phenotype correlation might hopefully aid in early diagnosis of this uncommon syndrome. We also strongly recommend early evaluation and assessment of airway so as to provide earlier and better treatment and prevention of any anticipated severe airway complications and thus looking forward to offering more comprehensive care for the patients and family support in the future.
Conflict of Interest
The authors have no conflict of interest to declare.
Author’s Contributions
Dr. Lun Chin Lin and Dr. Peir Taur Chen drafted the initial
manuscript.
Dr. Wan Hsin Wen, Dr. Ni-Chung Lee, Dr. En Ting Wu, Dr. Kai-Chi
Chang and Dr. Leticia B. Sy reviewed and revised the manuscript.
All authors contributed to acquisition of case details and the
analysis and interpretation of them.
All authors approved the final manuscript as submitted and
agree to be accountable for all aspects of the work.
Acknowledgement
We are also thankful to the little patient and his family for their kindness.
References
- Ivanovski I, Djuric O, Caraffi SG, Santodirocco D, Pollazzon M, et al. (2018) Phenotype and genotype of 87 patients with Mowat-Wilson syndrome and recommendations for care. Genet Med 20(9): 965-975.
- Stephanie H, Ho Ming L, Brian C, Jasmine F, Harriet M, et al. (2020) Mowat-Wilson syndrome in a Chinese population: A case series. Am J Med Genet A 182(6): 1336-1341.
- Yasunobu N, Ken O, Maki W, Takamasa Y, Yosuke K, et al. (2020) Successful treatment of drug-resistant status epilepticus in an adult patient with Mowat-Wilson syndrome: A case report. Epilepsy Behav Rep 14: 100410.
- Garavelli L, Ivanovski I, Caraffi SG, Santodirocco D, Pollazzon M, et al. (2017) Neuroimaging findings in Mowat-Wilson syndrome: a study of 54 patients. Genet Med 19(6): 691-700.
- Adam MP, Conta J, Bean LJH (2007) Mowat-Wilson syndrome. In: Adam MP, Ardinger HH, Pagon RA (Eds.), GeneReviews® University of Washington, Seattle, Washington, USA, 1993-2021.
- Garavelli L, Mainardi PC (2007) Mowat-Wilson syndrome. Orphanet Journal of Rare Diseases 2: 42.
© 2022 Peir Taur Chen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






