- Submissions

Full Text
Research in Pediatrics & Neonatology
The Genetic Architecture of CDKL5 Disorder in Childhood
Bittmann S*, Luchter E, Bittmann L, Alieva EM and Villalon
Department of Pediatrics, Ped Mind Institute (PMI), Germany
*Corresponding author: Stefan Bittmann, Department of Pediatrics, Ped Mind Institute (PMI), Germany
Submission: August 10, 2022; Published: August 29, 2022

ISSN: 2577-9200 Volume6 Issue5
Introduction
In 2004, a second gene was discovered whose mutations induce an atypical form of Rett syndrome, mutations in the CDKL5 gene. In the following period, it became clear that children with mutations in the CDKL5 gene have similar symptoms to classic Rett syndrome, but also differences, so it was stated to be treated as a neurodevelopmental disorder with an own entity [1-16]. CDKL5 disorder is defined as defect of the cyclin dependent kinase like 5 and defined as a difficult to treat epileptic encephalopathy [1-16]. Incidence varies from 1:40000-1:60000 [2-7, 9-16]. It is defined as early life difficult to treat epilepsy with abberant synaptic physiology. The CDKL5 protein is found in the cortex of the cerebrum, hippocampal areas like the fornix, the cerebellum, thalamic areas and the brain stem [4,5]. The female/ male distribution is predominant in females with a 4:1 ratio [1-16]. Females are most often heterozygote, males are hemizygote. Up to 270 different pathological variants of the CDKL5 gene are still described [15,16]. Point mutations, de novo-, missense and frameshift mutations were found [1-16]. The gene is found on 27 exons, whereas 6 are undefined and 21 expiring exons of the CDKL5 gene [2,3,6,8,16].
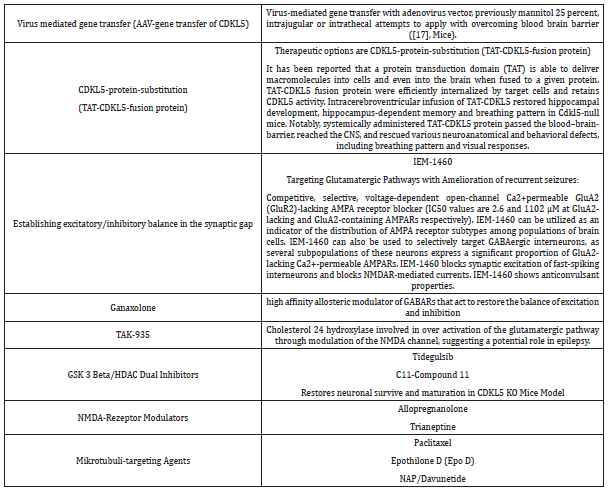
CDKL5 protein plays a major role in neuronal and dendritic growth and excitatory functions in the synaptic gap [1-16]. CDKL5 influences different pathways: the AKT-mTOR signaling pathway, the AKT/GSK 3 b signaling pathway, the BDNF-Rac-1 signaling pathway, the NGL-1-PSD 95 interaction and the microtubule development. CDKL5 Substrate in the brain are MeCP2, DNMT1, Rac1, Amphyphsin 1, PSD 95, NGL-1, Mind Bomb 1, Shoot in 1, HDAC 4 and IQGAP1 [3,6,7,9,12]. Symptoms are epileptic seizures, microcephalus, global developmental delay, speech developmental delay, autism, sleeping disorder, cortical visual impairment, hand stereotypies, gastrointestinal problems and SUDEP (sudden infant death in epilepsy) [1-16]. Patients with the CDKL5 gene defect show severe motor and cognitive developmental disorders shortly after birth. The majority of affected children develop severe epileptic seizures, among other symptoms [4,5]. Epileptic seizures generally begin by 6 weeks of age, and approximately 90% of affected CDKL5 patients struggle with these seizures daily [1-16]. Interesting new treatment approaches were summarized in detail in Table 1.
Table 1: Study Aspects of new therapy options for CDKL5 disorder in childhood.
Other commonly used treatment alternatives that CDKL5 patients have tried over the years include the ketogenic diet, onethird of CDKL5 patients have tried this method at least once. Vagus nerve stimulation have tried 20 per cent of all pediatric patients with CDKL5 disorder with different success. Treatment of epilepsy generally involves the use of 3 or more antiepileptic drugs with an average of 6 antiepileptic drugs. Other commonly used treatment alternatives that CDKL5 patients have tried over the years include the ketogenic diet. One-third of CDKL5 patients have tried this method at least once and vagus nerve stimulation. Approximately 20% CDKL5 patients have tried this method. However, epilepsy remains a serious complication for most people with CDKL5 disorder. Other commonly used treatment alternatives that CDKL5 patients have tried over the years include the ketogenic diet (onethird of CDKL5 patients have tried this method at least once) and vagus nerve stimulation (approximately 20% CDKL5 patients have tried this method). However, epilepsy remains a serious complication for most people with CDKL5 disorder.
Pharmacologic studies on Ataluren were initiated but only in nonsense mutation of the disease. Ataluren does not effectively cross the brain barrier. Fenfluramine is useful in Dravet syndrome and could be helpful in CDKL5 disorder, but its role is still not ruled out completely in pharmacological trials. Sabril (Vigabatrin), Valproat and Cortisone were frequently used in CDKL5 disorder patients. To establish excitatory and inhibitory balance in the synaptic gap to target the glutaminergic pathway, studies on NMDA and AMPAR blockers were initiated (IEM-1460, Ganaxolone, TAK- 935, Tidegusib, Compound-11, Allopregnanolone, Trianeptine). Nevertheless, CDKL5 protein substitution with TAT-CDKL5 fusion proteins are interesting research aspects for the future [1-16].
A few studies focus on deep brain stimulation of fornix structures of the hippocampus to restore hippocampal areas were evaluated and published. Nevertheless, green tea application, epigallocatechin-3-gallate, was used to restore CDKL5 dependent synaptical defects in vitro and in vivo. Microtubuli targeting agents like Paclitaxel, Epothilone D (Epo D) and NAP/Davunetide play a significant role in the disease and could diminish the degree of neuronal and dendritic impairment of CDKL5 gene dysfunction. Gene therapeutics cure the patient, but this is still in childhood shoes. The study by Gao et al. [17] published in Brain 2020, is a promising attempt to repair mutated genes by AAV transfer of CDKL5 [17]. Further studies in rodents and preclinical gene transfer studies must follow to develop the aspect of gene transfer to help the children with CDKL5 disorder to master their life without these incisive developmental complications in childhood.
References
- Jakimiec M, Paprocka J, Śmigiel R (2020) CDKL5 deficiency disorder-a complex epileptic encephalopathy. Brain Sci 10(2): 107.
- Demarest S, Pestana-Knight EM, Olson HE, Downs J, Marsh ED, et al. (2019) Severity assessment in CDKL5 deficiency disorder. Pediatr Neurol 97: 38-42.
- Wang HT, Zhu ZA, Li YY, Lou SS, Yang G, et al. (2021) CDKL5 deficiency in forebrain glutamatergic neurons results in recurrent spontaneous seizures. Epilepsia 62(2): 517-528.
- Kadam SD, Sullivan BJ, Goyal A, Blue ME, Hicks CS (2019) Rett syndrome and CDKL5 deficiency disorder: From bench to clinic. Int J Mol Sci 20(20): 5098.
- Siri B, Varesio C, Freri E, Darra F, Gana S, et al. (2021) CDKL5 deficiency disorder in males: Five new variants and review of the literature. Eur J Paediatr Neurol 33: 9-20.
- Demarest ST, Olson HE, Moss A, Knight EP, Zhang X, et al. (2019) CDKL5 deficiency disorder: Relationship between genotype, epilepsy, cortical visual impairment, and development. Epilepsia 60(8): 1733-1742.
- Kind PC, Bird A (2021) CDKL5 deficiency disorder: A pathophysiology of neural maintenance. J Clin Invest 131(21): e153606.
- Hao S, Wang Q, Tang B, Wu Z, Yang T, et al. (2021) CDKL5 deficiency augments inhibitory input into the dentate gyrus that can be reversed by deep brain stimulation. J Neurosci 41(43): 9031-9046.
- Chin RF, Mingorance A, Fell BR, Newell I, Evans J, et al. (2021) Treatment guidelines for rare, early-onset, treatment-resistant epileptic conditions: A literature review on dravet syndrome, lennox-gastaut syndrome and cdkl5 deficiency disorder. Front Neurol 12: 734612.
- Barbiero I, De Rosa R, Nielsen CK (2019) Microtubules: A key to understand and correct neuronal defects in CDKL5 deficiency disorder? Int J Mol Sci 20(17): 4075.
- Hong W, Haviland I, Knight EP, Weisenberg JL, Demarest S, et al. (2022) CDKL5 deficiency disorder-related epilepsy: A review of current and emerging treatment. CNS Drugs 36(6): 591-604.
- Dale T, Downs J, Olson H, Bergin AM, Smith S, et al. (2019) Cannabis for refractory epilepsy in children: A review focusing on CDKL5 deficiency disorder. Epilepsy Res 151: 31-39.
- MacKay CI, Bick D, Prokop JW, Muñoz I, Rouse J, et al. (2020) Expanding the phenotype of the CDKL5 deficiency disorder: Are seizures mandatory? Am J Med Genet A 182(5): 1217-1222.
- Olson HE, Costantini JG, Swanson LC, Kaufmann WE, Benke TA, et al. (2021) Cerebral visual impairment in CDKL5 deficiency disorder: Vision as an outcome measure. Dev Med Child Neurol 63(11): 1308-1315.
- Van Bergen NJ, Massey S, Stait T, Ellery M, Reljić B, et al. (2021) Abnormalities of mitochondrial dynamics and bioenergetics in neuronal cells from CDKL5 deficiency disorder. Neurobiol Dis 155: 105370.
- Pizzo R, Lamarca A, Pognetto MS, Giustetto M (2020) Structural bases of atypical whisker responses in a mouse model of CDKL5 deficiency disorder. Neuroscience 445: 130-143.
- Gao Y, Irvine EE, Eleftheriadou I, Naranjo CJ, Yeates FH, et al. (2020) Gene replacement ameliorates deficits in mouse and human models of cyclin-dependent kinase-like 5 disorder. Brain 143(3):811-832.
© 2022 Bittmann S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






