- Submissions

Full Text
Research in Pediatrics & Neonatology
Use of Monoclonal Antibodies Drugs Etesevimab and Bamlanivimab for the Treatment of Coronavirus Infection (COVID-19) in Adults and Children
Halyna Bulak1* and Andriy Zanevych2
1Associate Professor, Department of Pediatrics, Danylo Halytsky Lviv National Medical University, Ukraine
2Resident Doctor of Department of Vascular and Minimally Invasive Neurology and Neurosurgery, Danylo Halytsky Lviv National Medical University, Ukraine
*Corresponding author: Halyna Bulak, Associate Professor, Department of Pediatrics, Danylo Halytsky Lviv National Medical University, Ukraine
Submission: January 17, 2022; Published: February 16, 2022

ISSN: 2577-9200 Volume6 Issue3
Abstract
Coronavirus Disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus, which is a contagious respiratory virus causing atypical pneumonia COVID-19 in adult and children with Severe Acute Respiratory Syndrome (SARS). The SARS-CoV-2 genome also encodes four structural (S, E, M and N) and up to six accessory (3a, 6, 7a, 7b, 8, and 9b) proteins. The spike protein (S) is further divided into 2 subunits, S1 and S2 that mediate host cell attachment and invasion. Anti-SARS-CoV-2 Monoclonal Antibodies (mAbs) that target the spike protein have been shown to yield clinical benefits in treating SARS-CoV-2 infection. This article represents a clinical case of a 59-year-old man with Coronavirus Disease (COVID-19) and intracerebral haemorrhage, who was treated with the use of monoclonal antibodies bamlanivimab and etesevimab. Equally, it presents a review of these drugs’ application for COVID-19 treatment in adults and children.
Keywords: Coronavirus disease (COVID-19); Monoclonal Antibodies; Bamlanivimab; Etesevimab
Abbreviations: COVID-19: Coronavirus Disease 2019; mAbs: Monoclonal Antibodies; SARS: Severe Acute Respiratory Syndrome; FDA: US Food and Drug Administration; EUA: Emergency Use Authorization; RNA: Ribonucleic Acid; NIHSS: National Institutes of Health Stroke Scale; PCR: Polymerase Chain Reaction; CRP: C-Reactive Protein; CT: Computer Tomography; ESR: Erythrocyte Sedimentation Rate
Opinion
Coronavirus Disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. SARS-CoV-2 is a contagious respiratory virus causing atypical pneumonia COVID-19 in adults and children with Severe Acute Respiratory Syndrome (SARS). Most people infected with the virus will experience mild to moderate respiratory illness and recover without requiring special treatment. However, some will become seriously ill and require medical attention. As of December 14, 2021, more than 270 million cases of COVID-19-caused by SARS-CoV-2 infection-have been reported globally, including more than 5.3 million deaths [1]. SARS-CoV-2 has a single-stranded, positive-sense RNA (+RNA). The SARS-CoV-2 genome also encodes four structural (S, E, M, and N) and up to six accessory (3a, 6, 7a, 7b, 8, and 9b) proteins, but their translation requires newly synthesized individual Subgenomic RNAs (sgRNA) in the infected cells [2]. The spike protein (S) is further divided into 2 subunits, S1 and S2, that mediate host cell attachment and invasion. Through its Receptor-Binding Domain (RBD), S1 attaches to Angiotensin-Converting Enzyme 2 (ACE2) on the host cell; this initiates a conformational change in S2 that results in virus-host cell membrane fusion and viral entry [3]. Anti-SARSCoV- 2 Monoclonal Antibodies (mAbs) that target the spike protein have been shown to yield clinical benefits in treating SARS-CoV-2 infection. Nowadays, the following monoclonal antibodies are employed in the treatment of mild to moderate COVID-19: bamlanivimab and etesevimab, casirivimab plus imdevimab, sotrovimab.
Case Report
Described below is a medical case of a 59-year-old man with Coronavirus Disease (COVID-19) and intracerebral haemorrhage. At the end of November 2021, the patient was hospitalized to Drohobych Central Regional Hospital in severe condition. According to relatives, the patient developed speech disorders and weakness in the right extremities. The deterioration was due to stress. Objectively, when hospitalized: severe condition. The cutaneous integuments were pink. Vitals: arterial pressure on the arm-160/110mmHg, heart rate of 78 beats/minute, heart tones are muffled, respiratory rate of 18 breaths/minute. The abdomen was soft, painless, available for palpation in all sections, no abdominal distension was detected. No edema. Neurological examination: The patient was conscious, did not perform instructions, inhibited. There were also observed elements of global aphasia and episodes of psychomotor arousal. The pupillary response was retained D=S. Eye movements were normal. His face was asymmetric in right part. Smoothed right nasolabial fold. The tongue was midline with normal movements and no atrophy, swallowing saved. Reflexes D>S. The medical examination revealed right-sided deep hemiparesis. Babinski reflex was positive in the right foot, while sensory disorders were difficult to diagnose. Meningeal signs: neck stiffness +1.5sm, Kernig’s signs negative. The patient was consulted by a neurologist. The doctor performed a CT scan of the brain, which revealed a massive intracerebral hemorrhage in the left hemisphere. The patient was hospitalized in the intensive care unit. Among laboratory tests: complete blood count, blood glucose test, coagulogram, blood biochemistry, urinanalysis were performed. High cholesterol level was detected. The patient was consulted by a neurosurgeon, ophthalmologist, therapist. Received treatment: saline, mannitol, furosemide, diazepam, dexketoprofen, analgin. The patient’s condition has improved - he understood the language addressed, answered the questions in words. Right-sided hemiparesis decreased. Reflexes D>S. Neck stiffness preserved. According to the NIHSS scale-10 points. On the 9th-10th day of treatment, the patient’s temperature rose to 37-38 °C, there were complaints of pain and sore throat. A rapid test for Sars-Cov-2 antigen turned out positive.
On the 11th day after the onset of stroke and the 2nd day of fever, the patient was transferred for further treatment to the COVID-zone of the Department of Therapy of 1st Territorial Medical Association of Lviv. At the time of hospitalization, the patient’s condition was severe. Vitals: vesicular breath sounds, without wheezing, respiratory rate of 16 breaths/minute, arterial pressure on the arm- 150/90mmHg, heart rate of 78 beats/minute, a body temperature of 37,5 °C, SpO2-95-96% on room air. Neurological examination: The patient was conscious, alert, performed instructions, with elements of expressive aphasia, emotionally unstable. The pupillary response was retained D=S. Eye movements were within the range of normal. His face was asymmetric in right part. Smoothed right nasolabial fold. Tongue was midline with normal movements and no atrophy. Swallowing saved. Reflexes D>S. Right-sided hemiparesis and hemianesthesia were detected. Babinski reflex was positive in the right foot. Meningeal signs were absent. Computer tomography of the brain was performed repeatedly. The CT scans showed subacute intracerebral hemorrhage in the left hemisphere. Chest X-ray was performed as well: without visible focal-infiltrative changes (Figure 1a). The patient’s PCR test for COVID-19 was positive. The final diagnosis was: Coronavirus Disease (COVID-19). Intracerebral hemorrhage in the left hemisphere. Hypertensive Heart Disease. Among laboratory tests: complete blood count, blood glucose test without pathological changes.
Figure 1: Chest X-Ray on the second day(а) and on the 11th day(b) after hospitalization.
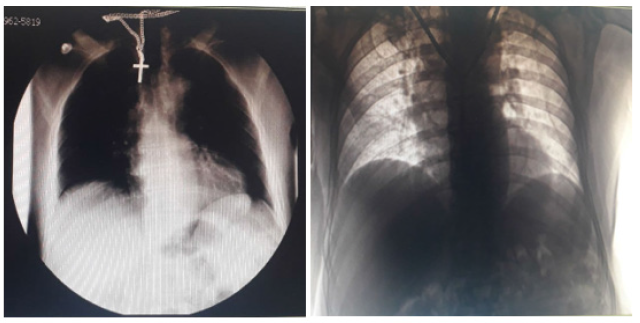
Bamlanivimab 700mg and etesevimab 1400mg per 400ml of 0.9% NaCl were administered as an Intravenous (IV) infusion during 60-70 minutes on the 2nd day after hospitalization and the 4th day after the onset of COVID-19 symptoms. Mannitol, Ringer’s solution, saline, magnesium sulfate, paracetamol were also prescribed. The blood biochemistry and coagulogram revealed a slightly elevated level of creatine and urea. D-Dimer Test and C-Reactive Protein Test were held, the results: D-Dimer-8927,8ng/ ml FEU, CRP-54mg/ml, when normal values are D-Dimer-<500ng/ ml FEU, CRP -<6mg/ml. The patient’s condition remained stable, SpO2 - 96-98% on the room air, the body temperature came back to normal on the 5th day, he began to speak in sentences, weakness in the right extremities decreased. Chest X-Ray was conducted on the 11th day after hospitalization: unconvincing signs of left lower lobe pneumonia (Figure 1b). Blood test detected an elevated Erythrocyte Sedimentation Rate (ESR), C-reactive protein - 54mg/ ml (normal-<6mg/ml), D-dimers-11067.8ng/ml FEU (normal- <500ng/ml FEU). Subcutaneous injections of enoxaparine sodium 0.2ml (20mg) 2 times per day were added to treatment due to the increased level of D-dimers. The second PCR test came up negative on the 14th day after hospitalization.
For further treatment, the patient was transferred to the Department of Vascular and Minimally Invasive Neurology and Neurosurgery 1st Territorial Medical Association of Lviv. CT of the brain and chest revealed intracerebral hemorrhage in the resorption stage, subatelectasis S10 of the right lung. The patient received the following treatment: Ringer’s solution, saline, enoxaparin sodium injection of 0,4mg 1 time per day. The patient’s condition was stable with improvement, he was able to sit unaided, mild right-sided hemiparesis persisted, his speech improved significantly, the body temperature-36,6-37,2 °C, SpO2-96-98%. The patient worked with a rehabilitation specialist and a speech therapist. The screening included complete blood count, blood glucose test, coagulogram, blood biochemistry, urinanalysis, D-Dimer Test. Elevated ESR level was detected-44 (norm for people-1-10) along with low content of leukocytes in urine, the level of D-dimers decreased to 2216.2ng/ ml FEU. The patient was discharged home with improvement on the 27th day of hospitalization. It was recommended to continue training with a rehabilitation and speech therapist, monitor blood pressure, take enalapril 5mg and aspirin 100mg constantly.
Discussion
Patients with SARS-CoV-2 infection can be classified into 5 groups according to the severity of disease: asymptomatic infection (individuals who test positive for SARS-CoV-2 using a virologic test, but who have no symptoms that are consistent with COVID-19), mild illness (patients who have any of the various signs and symptoms (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell), moderate illness (patients who show evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO2) ≥94% on room air at sea level), severe illness (individuals who have SpO2 <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/ FiO2) <300mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates >50%), critical illness (patients who have respiratory failure, septic shock, and/or multiple organ dysfunction) [4].
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) to permit the emergency use of the unapproved products bamlanivimab and etesevimab administered together for the treatment of mild to moderate Coronavirus Disease 2019 (COVID-19) in adults and pediatric patients, including neonates, with positive results of direct SARSCoV- 2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death [5]. For treatment of COVID-19, bamlanivimab and etesevimab should be administered together as soon as possible after positive results of direct SARSCoV- 2 viral testing and within 10 days of symptom onset [5].
Bamlanivimab and etesevimab are not authorized for use in adults and pediatric patients 2 years and older who are hospitalized due to COVID-19. The reasons for hospital admission may be different and the threshold for hospital admission may be lower for neonates, young infants, and toddlers with COVID-19 compared to older children and adults. The authorization covers young children (i.e., birth to 2 years of age) who are hospitalized with mild to moderate COVID-19 at the time of treatment to receive bamlanivimab and etesevimab [6].
Bamlanivimab and etesevimab are not authorized for use in
patients, regardless of age, who:
A. Require oxygen therapy and/or respiratory support due
to COVID-19, OR
B. Require an increase in baseline oxygen flow rate and/or
respiratory support due to COVID-19 and are on chronic oxygen
therapy and/or respiratory support due to underlying non-
COVID-19 related comorbidity [6].
The authorized dosage for adults (18 years and older) and pediatric patients (<18 years and weighing at least 40kg) is 700mg bamlanivimab and 1,400mg of etesevimab administered together as a single Intravenous (IV) infusion. The authorized dosage for pediatric patients weighing less than 40kg will vary depending on weight [5]. There exist multiple surveys confirming the efficacy of these monoclonal antibodies for COVID-19 treatment among nonhospitalized patients, in adults as well as in children.
769 non-hospitalized adults with mild-to-moderate COVID19 symptoms who were at high risk for progression to severe COVID-19 participated in the phase 3 portion of a randomized, double-blind, placebo-controlled clinical trial (BLAZE-1). Of these patients, 511 received a single infusion of bamlanivimab 700 mg and etesevimab 1,400mg together and 258 received placebo. Hospitalization or death occurred in 15 (6%) patients who received placebo compared to 4 (0.8%) patients treated with bamlanivimab 700 mg and etesevimab 1,400mg administered together (p<0.001), an 87% reduction. All deaths (n = 4, 1.6%) occurred in the placebo group. In addition, patients that received bamlanivimab 700mg and etesevimab 1,400mg together had a faster time to symptom resolution in the clinical trial [7].
125 non-hospitalized children with mild-to-moderate COVID-19 symptoms took part in in the Phase 3 portion of BLAZE-1. Pediatric patients weighing 40kg or more received the same dose as adults, while those weighing less than 40kg received weightbased dosing. Of the 125 pediatric subjects, 33 subjects ages 12 to <18 were evaluated in double-blind, placebocontrolled Phase 3 cohorts of BLAZE-1, and 1 subject age 12 to <18 was evaluated in a controlled addendum to BLAZE-1. Of the 33 childrens, 14 received placebo, 14 received the authorized dose or a higher dose for their age, and 5 received a lower dose for their age. A total of 91 pediatric subjects were evaluated in an open-label addendum to BLAZE-1 [5]. The youngest participant in the trial was 10 months of age and weighed 8.6kg. None of the children died or were hospitalized, but there was detected no significant efficacy compared to placebo due to the limited number of pediatric patients in the trial.
The FDA EUAs do permit the use of these agents in patients who are hospitalized for a diagnosis other than COVID-19, provided they have mild to moderate COVID-19 and are at high risk for progressing to severe disease [5,6]. Treatment with bamlanivimab and etesevimab has not been studied in patients hospitalized due to COVID-19. A substudy of the ACTIV-3 trial randomized patients who were hospitalized for COVID-19 to receive bamlanivimab 7,000mg or placebo, each in addition to remdesivir. On October 26, 2020, study enrollment was halted after a prespecified interim futility analysis indicated a lack of clinical benefit for bamlanivimab [8]. Therefore, the study of the use of monoclonal antibodies in hospitalized patients with mild to moderate COVID-19 who are at high risk of disease progression due to comorbidities and other factors looks promising and requires f9urther clinical trials.
Conclusion
All things considered, the use of monoclonal antibodies for the treatment of coronavirus infection in adults and children is one of the promising areas in the prevention and treatment of COVID-19. Bamlanivimab and etesevimab reduce hospitalizations and deaths from COVID-19 in both non-hospitalized adults and children, as demonstrated by clinical trials. These drugs have proven effective in the treatment of mild-to-moderate COVID-19 after positive results of direct SARS-CoV-2 viral testing and within 10 days of symptoms onset. The study of the use of monoclonal antibodies in hospitalized patients with mild to moderate COVID-19 who are at high risk of disease progression due to comorbidities and other factors appears to be perspective and requires further clinical trials.
References
- (2021) COVID-19 Dashboard by the center for science and engineering, Johns Hopkins University and Medicine, USA.
- Brant AC, Tian W, Majerciak V, Yang W, Zheng ZM (2021) SARS-CoV-2: From its discovery to genome structure, transcription, and replication. Cell Biosci 11(1): 136.
- Jiang S, Hillyer C, Du L (2020) Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol 41(5): 355-359.
- (2019) COVID-19 treatment guidelines panel, Coronavirus Disease 2019 (COVID-19) Treatment guidelines. National Institutes of Health, Maryland, USA.
- (2021) Fact sheet for healthcare providers: Emergency Use Authorization (EUA) of bamlanivimab and etesevimab, Food and Drug Administration, Maryland, USA.
- (2021) Frequently asked questions on the emergency use authorization for bamlanivimab and etesevimab, Food and Drug Administration, Maryland, USA.
- Dougan M, Azizad M, Mocherla B, Gottlieb RL, Chen P, et al. (2021) A randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with covid-19 and validation of the prognostic value of persistently high viral load. Clin Infect Dis.
- (2020) Statement-NIH-sponsored ACTIV-3 trial closes LY-CoV555 sub-study, National Institute of Allergy and Infectious Diseases, Maryland, USA.
© 2022 Halyna Bulak. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






