- Submissions

Full Text
Research in Pediatrics & Neonatology
Analysis of Cytokines in Patients with Juvenile Idiopathic Arthritis
Huber AL and Brunner J*
Department of Pediatrics, Medical University Innsbruck, Austria
*Corresponding author: Brunner J, Department of Pediatrics, Medical University Innsbruck, Austria
Submission: November 19, 2021; Published: February 08, 2022

ISSN: 2577-9200 Volume6 Issue2
Abstract
Introduction: Juvenile Idiopathic Arthritis (JIA) is the most common autoimmune disease in childhood.
It is defined as arthritis in one or more joints in children with an age before 16 years. There are several
subgroups of JIA. Aims of the study were to assess whether or not the cytokines IL-4, IL-10, IL-17, MCP-1
and TNF-α are elevated in the active stadium of the disease and whether or not there are patterns which
are specific for the different subgroups of JIA.
Methods: 148 plasma and serum samples of 58 JIA patients with an average age of 11.3±5 years have
been examined, as well as 118 plasma and serum samples of 118 controls or Normal Healthy Donors
(NHD) with an average age of 34.9±16.8 years. The subgroups of JIA were defined as Persistent Oligoarticular
JIA (PO JIA), Extended Oligoarticular JIA (EO JIA), Polyarthritis Rheuma Factor Positive JIA
(RF+JIA), Enthesitis Related Arthritis (ERA JIA), Polyarthritis Rheuma Factor Negative JIA (RF-JIA) and
Psoriasis Arthritis JIA (PS JIA). The serum levels of cytokines IL-4, IL-10, IL-17, MCP-1 and TNF-α were
determined using ELISA technique.
Results: The levels of all measured cytokines were elevated in JIA patients compared to normal healthy
donors. With an average value of 5.2±6.1pg/ml, IL-4 was significantly higher in JIA patients compared to
controls, with an average value of 1.3±1.2pg/ml (p=0.0182; Mann Whitney test). However, the differences
regarding the other cytokines were statistically not significant. The ROC analysis of IL-4 revealed an area
under the curve of 0.7744 (p=0.0222); at a cut-off point of >1.68pg/ml IL-4 showed to have the highest
sensitivity of 76.32 and a specificity of 71.43 for distinction of JIA from controls. The different cytokines
were specifically elevated in different subgroups of JIA, but, however in no case statistically significant:
IL-4 was elevated in PO JIA, EO JIA, RF+JIA, and RF-JIA; TNF-α was elevated in EO JIA and RF+JIA; MCP-1
was elevated in PO JIA and RF+JIA; IL-10 was elevated in PO JIA, EO JIA, RF+JIA and IL-17 was elevated
in RF+JIA and RF-JIA. Interestingly, no cytokine was found to be elevated in ERA JIA or PS JIA patients. All
cytokines tested were elevated in patients with active disease.
Conclusion: All parameters tested showed systemically higher values in JIA patients compared to controls:
The most pronounced affect was found in IL-4. Different, specific elevation patterns were found
in the subgroups of JIA. The marked elevation of IL-17 and IL-4 suggest treatment trials using specific
antibodies, e. g. Ixekizumab or Dupilumab.
Keywords: Jucenile idiopathic arthritis; Cytokines; IL-4; IL-10; IL-17; MCP-1; TNF- α
List of Abbreviations: A: Active Phase; ANAs: Anti-Nuclear Antibodies; AUC: Area Under The Curve; CCL/
CXCL: Chemokine Ligand; CD8+T Cell: Cytotoxic T Cell; CHAQ: Childhood Health Assesment Questionnaire;
CRP: C-Reactive Protein; DMARDs: Disease-Modifying Antirheumatic Drugs; ELISA: Enzyme-linked
Immunosorbant Assay; EO JIA: Extended Oligoarticular Juvenile Idiopathic Arthritis; ERA: Enthesitis-Related
Arthritis; ESR: Erythrocyte Sedimentation Rate; f: Female; HLA: Human Leukocyte Antigen; IAC:
Intra-Articular Corticosteroid; Ig: Immunoglobulin; IL: Interleukin; JIA: Juvenile Idiopathic Arthritis; m:
Male; Mb.: Morbus; MCP-1: Monocyte Chemoattractant Protein-1; MTX: Methotrexate; NA: Remission;
NHD: Normal Healthy Donor; NK-Cells: Natural Killer-Cells; ns: Not Significant; NSAIDs: Nonsteroidal
Anti-Inflammatory Drugs; PO JIA: Persistent Oligoarticular Juvenile Idiopathic Arthritis; PS JIA: Psoriatic
Juvenile Idiopathic Arthritis; RANKL: Receptor Activator of NF-κB Ligand; RF+: Rheumatoid Factor
Positive; RF-: Rheumatoid Factor Negative; ROC: Receiver-Operating-Characteristic-Curve; SJIA: Systemic
Juvenile Idiopathic Arthritis; TH: Triamcinolone Hexacetonide; Th-Cells: T-Helper-Cells; TNF: Tumor Necrosis
Factor
Introduction
Juvenile Idiopathic Arthritis (JIA) is the most common autoimmune disease in childhood [1]. It is defined as arthritis in one or more joints in children with an age before 16 years. Symptoms last at least six weeks [2]. In most cases JIA presents with peripheral arthritis. By including other symptoms as well as various diagnostic parameters it is divided into several subgroups, these are listed in more detail below [1].
Classification Into Subsets
Polyarticular JIA
Polyarticular JIA is defined by more than four affected joints in the first six months of disease onset [3]. Females are more frequently affected than males [4]. Disease onset starts under the age of 16 years [3]. There are seen two peaks of onset: one between the age of two and five years and the second one between ten and 14 years [4]. Patients can be divided into Rheumatoid Factor Positive (RF+) or Rheumatoid Factor Negative (RF-) groups [3]. RFJIA is more often seen in patients than RF+ JIA. RF+ JIA normally affects first smaller joints symmetrically, and then also larger joints. RF- JIA affects both large and small joints asymmetrical [5]. Uveitis as an extra-articular manifestation is observed in polyarticular RFpatients [6].
Oligoarticular JIA
Oligoarticular JIA is defined as JIA with four or less than four affected joints in the first six months of disease onset [7]. It is the most common subgroup of JIA, and it affects females more often than males [8]. If the number of affected joints does not increase after the first six months, it is called Persistent Oligoarticular JIA (PO JIA). If the number of affected joints increases to over four after six months, it is called Extended Oligoarticular JIA (EO JIA) [7]. If there is only one joint affected, it is called monoarthritis [9]. This disease normally starts at the age before six years. Moreover, large joints are affected asymmetrically [5]. Importantly, regular eye-checks are necessary because of a high risk to develop chronic iridocyclitis [10].
Enthesitis-related arthritis
Children with Enthesitis-Related Arthritis JIA (ERA JIA) present with enthesitis, a combination of enthesitis and arthritis, juvenile ankylosing spondylitis or with inflammatory bowel disease. Most affected joints at onset are the knees, the sacroiliacs, ankles and hips, small joints of the feet and toes. Boys are more often affected than girls. Normally, disease onset occurs in late childhood with a peak at the age of twelve [2]. Half of the patients are tested Human Leukocyte Antigen-B27 (HLA-B27) positive. In 20 % a positive family history of HLA-B27 associated diseases can be found [11]. Furthermore, HLA-B27 is associated with acute anterior uveitis [12].
Morbus (Mb.) still or Systemic JIA (SJIA)
Besides of arthritis, systemic JIA patients present with extra-articular symptoms like fever, rash, hepatosplenomegaly, lymphadenopathy and serositis. Both females and males are affected with the same symptoms [13]. In most of the patients’ systemic symptoms decline after some months. Half of the patients recover nearly completely while in the other half joint involvement continues [14]. Most affected joints are the knees, wrists, ankles and also small joints of hand and foot, elbow, shoulder, hip, temporomandibular joint and cervical spine. The clinical course can be variable: monocyclic with a single episode of illness and remission, polycyclic with active disease episodes and times of quiescence and persistent, which is associated with arthritis and sometimes with further systemic symptoms [13].
Psoriatic JIA
Psoriatic JIA (PS JIA) is defined as arthritis with psoriasis. The most common other symptoms are dactylitis, nail pitting and onycholysis. Females are more often affected than males [15]. There are two peaks of onset, the first one occurs during the preschool years and the second one during middle to late childhood [16]. More than 50 % of the patients are tested positive for Anti-Nuclear Antibodies (ANAs) [15]. Arthritis affects one (monoarthritis; especially involvement of the knee) or more joints (oligo- or polyarthritis), axial and peripheral ones [15,16]. Symptoms of arthritis can start years earlier than the symptoms of psoriasis. Furthermore, there is also a high risk of developing chronic uveitis [15]. In many cases psoriasis or arthritis are running in the family [17].
Unclassified JIA
Children with unclassified JIA cannot get assigned to any of the subgroups above because they can get assigned to more than one of these subgroups or to no one [18].
Diagnosis
There are no specific laboratory examinations parameters for JIA. Diagnosis therefore is based on clinical presentation. JIA laboratory data can be helpful. There are different possibilities for measuring the disease activity for instance patient visual analogue scale, physician visual analogue scale, number of active joints, anaemia, ESR, thrombocyte count, Steinbrocker score, but none of these tests is totally accurate. JADAS score? For physical, social, and mental functioning Childhood Health Assesment Questionnaire (CHAQ) and Paediatric Quality of Life Inventory were established [1].
Cytokines
IL-4 belongs to the family of cytokines and is part of the specific immune response. It is required for the modification of T-helper cells to T-helper2-cells (Th2-cells) and inhibits activation of macrophages through TNF-gamma. It also inhibits the secretion of pro-inflammatory chemokines and cytokines in macrophages [19,20]. IL-4 is also required for immunoglobulin isotype switching of Immunoglobulin G (IgG) and Immunoglobulin M (IgM) to Immunoglobulin E (IgE). So, IL-4 plays a regulatory role in allergic response as well, as it is a growth factor for mast cells since these involve IgE-mediated mast cell degranulation [21-24].
IL-10 is also an anti-inflammatory cytokine and prevents inflammatory and autoimmune pathologies. Most IL-10 gets derived out of Th-cells, monocytes, macrophages, and dendritic cells, but also out of cytotoxic T-cells, natural killer-cells (NK-cells),mast cells and granulocytes. When there is an infection or tissue damage also epithelial cells and keratinocytes can release IL-10. Deficiency or aberrant expression of IL-10 can lead to autoimmune diseases, inflammatory bowel diseases and any other inflammatory response. IL-10 plays an important role in mediating host antiinflammatory response, prevents damage to the host and keeps up normal tissue homeostasis [25].
Moreover, IL-17 is also a pro-inflammatory cytokine. It is mainly produced by T-helper17 cells (Th17-cells), but also by adaptive and immune cell types, including cytotoxic T cells (CD8+T cells), NK-cells and innate lymphoid cells and neutrophiles. During inflammation, IL-17 induces granulopoieses factors and neutrophile-specific chemokines like chemokine ligand 1 (CXCL1), CXCL2, CXCL5, CXCL8, mediators of the acute phase response like IL-6, the pro-inflammatory cytokines which include tumor necrosis factor, IL-1β, and Receptor Activator of NF-κB Ligand (RANKL) and matrix metalloproteinases. IL-17 cannot combat an inflammation, but in cooperation with TNF and IL-17 an upregulation of target genes is possible [26,27]. Monocyte Chemoattractant Protein-1 (MCP-1), or chemokine ligand 2 (CCL2), is a chemokine and belongs to the family of cytokines. CCL2 is produced by many cell types: endothelial, fibroblasts, epithelial, smooth muscle, mesangial, astrocytic, monocytic, and microglial cells. CCL2’s major sources are monocytes/macrophages. As a proinflammatory cytokine it is involved in recruiting monocytes, neutrophils, and lymphocytes. It plays a major role in regulation of migration and infiltration of monocytes/macrophages, memory T lymphocytes, NK-cells and induces chemotaxis. “Migration of monocytes from the blood stream across the vascular endothelium is required for routine immunological surveillance of tissues, as well as in response to inflammation [28].
TNF-α belongs to the tumor necrosis factor superfamily. It is a proinflammatory cytokine. Mainly secreted by macrophages/ monocytes during an acute inflammation, it is involved in the regulation of cell proliferation, differentiation, necrosis, apoptosis, lipid metabolism and coagulation. It is also important for resistance to infection and cancers [29,30]. In this study we compared IL-4, IL-10, IL-17, MCP-1 and TNF alpha between a group of 58 patients and 118 controls. The patient group included children which were affected by JIA. The JIA-affected children were classified in the following subgroups: oligoarticular JIA, polyarticular JIA, arthritis associated with enthesitis, systemic JIA or Mb. Still, psoriatic JIA and unclassified JIA. The control group consisted of Normal Healthy Donors (NHD) who were not affected by any rheumatic or feverish disease.
Aim of this study was to evaluate whether there is any significant difference between JIA patients and controls regarding the laboratory markers IL-4, IL-10, IL-17, MCP-1 and TNF-alpha. Second, it was analyzed whether there is any difference within the JIA subgroups in the mentioned markers. Third, it was analyzed whether there is any prognostic trend in the laboratory markers of the JIA patients. Fourth, it was analyzed if there is any difference within the cytokines in active phase of JIA or remission.
Ethics
An ethics applicant with title “Biomarker bei Autoimmunopathien in der Pädiatrie” (Biomarker in paediatric autoimmune diseases) has been granted in 2009. The applicant contained the information forms for patients from age lessthan eight until age 18, information forms for the parents of the children and information forms for adult test persons. At the meeting of the ethics committee of the medical university Innsbruck on 27.08.2009 the ethics applicant got grant with file reference UN3731//280/4.5.
Methods and Material
148 plasma and serum samples of 58 JIA patients have been taken, as well as 118 plasma and serum samples of 118 controls. The control group consisted of NHD. Due to ethics most of NHD were adult persons. The plasma and serum samples of the JIA patients group consisted of 50 samples of 21 patients which built up the subgroup Persistent Oligoarticular JIA (PO JIA), of 21 samples of eight patients which built up the subgroup Extended Oligoarticular JIA (EO JIA), of 24 samples of 4 patients which built up the subgroup polyarthritis Rheuma Factor Positive JIA (RF+JIA), of 10 samples of 4 patients which built up the subgroup Enthesitis Related Arthritis (ERA JIA), of 38 samples of 11 patients which built up the subgroup Polyarthritis Rheuma Factor Negative JIA (RF-JIA) and of 5 samples of 3 patients which built up the subgroup Psoriasis Arthritis JIA (PS JIA). Time of acceptance has been the visitation of the outpatient department. Either serum or plasma samples were taken, which had been frozen after separation from corpuscular blood elements by centrifugation. At a later stage, after completion of all samples of all patients, the quantification of the different cytokines has been performed using standard Enzyme-Linked Immunosorbent Assay (ELISA). In order to guarantee the homogeneity of the measurements, the ELISA-tests had been performed on one single day, with one homogenous batch of ELISA-kits for each cytokine, respectively.
Method
The double antibody sandwich technique was used for measuring the cytokines MCP-1, TNF-α, IL-4, IL-10 and IL-17 in this study. This test for measuring the concentration of antigens utilizes polystyrene microtiter plates as solid phase basis coated with the specific antibodies. The specific antigen (in the specimens) is added and binds to the specific antibodies. After a washing step to remove the supernatant, another second antibody, coupled with an enzyme is added, which binds to the antigen as well. A colored substrate is added ultimately, which binds to the enzyme, and can be measured photo-metrically now. The amount which is measured is proportional to the amount of antigen bound to the first antibody [31].
Statistics
Descriptive statistics were done by means of the Excel programme (Microsoft Corp., Redmont, Washington, USA) and Graph Pad Prism version 7 (GraphPad Software, La Jolla, California, USA). First, data was analyzed for normal distribution. For this task, the d’Agostino & Pearson and Shapiro & Wilk tests have been used. Because of the small size of the groups, and partial non-Gaussian distributions of the values, nonparametric tests were used. Comparisons of two groups have been conducted by the U-test of Mann and Whitney, while comparisons of more than two groups have been conducted by the nonparametric test of Kruskal and wallis, combined with the posthoc test after Dunn, in order to correct for multiple testing. The nonparametric method of spearman has been used for correlation analysis. All tests were performed two sided. A p value below 0.05 was considered statistically significant.
Results
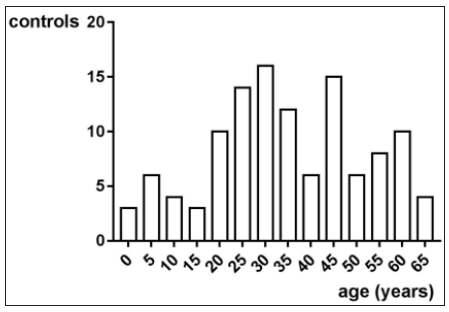
In this trial 58 patients and 118 controls have been enrolled. Altogether, the average age was 19.1±15.1 years. There have been taken 148 samples of 58 JIA patients with an average age of 11.3±5 years. Figure 1 shows the age distribution. There have been taken 118 samples of 118 controls with an average age of 34.9±16.8 years. Figure 2 shows the age distribution. The average age of the girls with 19.4±15.4 years does not differ from the average age of the boys with 23.1±17.7 years (p>0.05, Mann Whitney test).
Figure 1: The average age of all specimens of all JIA Patients is 11.3±5 years. The distribution is not Gaussian, with a negative skew.
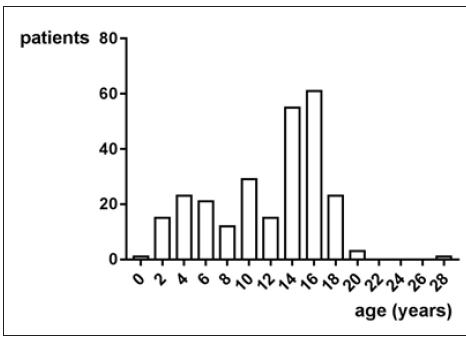
Figure 2: The average age of all controls is 34.9±16.8 years. The distribution is not Gaussian according to the normality tests of D’Agostino and Pearson and Shapiro-Wilk (p<0.05 each).
MCP-1
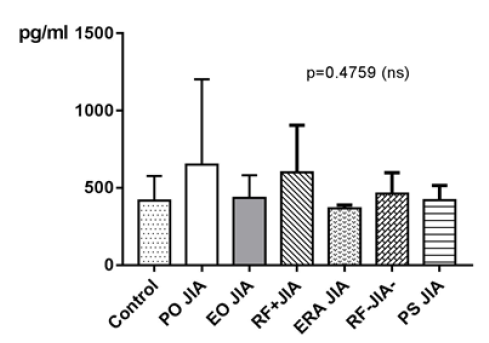
MCP-1 in JIA patients shows an average value of 551.2±377.6pg/ ml and is therefore higher than in the controls, which shows an average value of 417.8±159.4pg/ml. With p=0.2765 (Mann Whitney test) the difference between JIA patients and controls is statistically not significant (Figure 3). Comparing all the subgroups and the controls with each other shows that the average values of MCP-1 in patients with PO JIA and in patients with RF+JIA are higher than the values of MCP-1 in the controls and are also higher than in other subgroups. Both also show a higher standard deviation than the other compared groups (Figure 4).
Figure 3: MCP-1 in pg/ml at the y-axis (axis of ordinates) and divided into JIA patients and controls. The difference is statistically not significant (Mann Whitney test).

Figure 4: MCP-1 in pg/ml at the y-axis (axis of ordinates) and divided into the subgroups Control, Persistent Oligoarticular JIA (PO JIA), Extended Oligoarticular JIA (EO JIA), Polyarthritis Rheuma Factor Positive JIA (RF+JIA), Enthesitis Related Arthritis JIA (ERA JIA), Polyarthritis Rheuma Factor Negative JIA (RF-JIA), Psoriasis Arthritis JIA (PS JIA). The differences in between the groups are statistically not significant (p>0.05; Kruskal Wallis test and Dunn’s posthoc test).
MCP-1 with a Spearman ρ of -0.03897 does not correlate statistically significant with the age (p=0.7348).
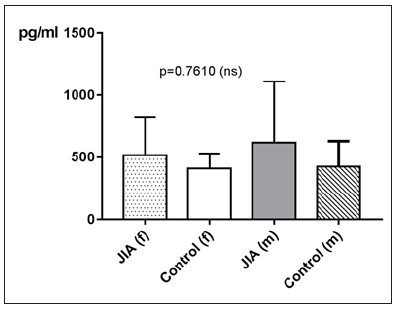
Regarding gender differences in MCP-1 between JIA patients and controls, this groups are divided into JIA Female (JIAf) and JIA Male (JIAm) and controls female (Controlf) and controls male (Controlm). With p=0.7610 the differences in between the groups are statistically not significant. The Receiver-Operating- Characteristic-Curve (ROC) analysis of MCP1 shows a line paralleling the line of identity, which is not significant (Figure 5).
Figure 5: MCP-1 in pg/ml at the y-axis (axis of ordinates) and divided into JIAf, Controlf, JIAm and Controlm. The differences in between the groups are statistically not significant (p>0.05, Kruskal Wallis test).
TNF-α
TNF-α in JIA patients shows an average value of 17.9±13pg/ ml and is therefore higher than in the controls, which shows an average value of 13±4.9pg/ml. With p=0.7090 (Mann Whitney test) the difference between JIA patients and controls is statistically not significant (Figure 6). Comparing all the subgroups and the controls with each other shows, that the average values of TNF-α in patients with PO JIA, EO JIA, RF+JIA and in patients with PS JIA are higher than the values of TNF-α in the controls and also higher than in other subgroups. They also show a higher standard deviation than the other compared groups (Figure 7). TNF-α with Spearman ρ=- 0.2031 and p=0.0708 correlates slightly negative, but statistically not significant with the age (Figure 8). However, the p value of 0.07 indicates a trend: the higher the age, the lower the TNF-α. Regarding gender differences in TNF-α between JIA patients and controls, this groups are divided into JIA Female (JIAf) and JIA Male (JIAm) and Controls Female (Controlf) and Controls Male (Controlm). With p=0.1961 the differences in between the groups are statistically not significant. The ROC analysis of TNF-α shows a line paralleling the line of identity, which is not significant (Figure 9).
Figure 6: TNF-α in pg/ml at the y-axis (axis of ordinates) and divided into JIA patients and controls. The difference is statistically not significant.
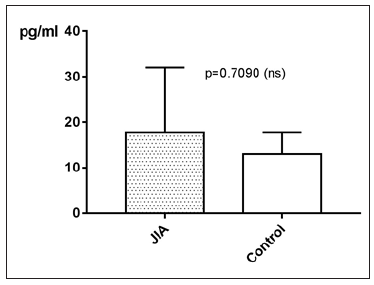
Figure 7: TNF-α in pg/ml at the y-axis (axis of ordinates) and divided into the subgroups control, PO JIA, EO JIA, RF+JIA, ERA JIA, RF-JIA, PS JIA. The differences in between the groups are statistically not significant (p>0.05 Kruskal Wallis test and Dunn’s posthoc test).
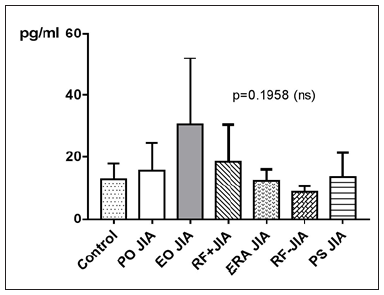
Figure 8:TNF-α in pg/ml at the y-axis (axis of ordinates) with the age in years at the x-axis (x-coordinate). The correlation of TNF-α and the age is statistically not significant (spearman ρ= -0.2031, p=0.0708).
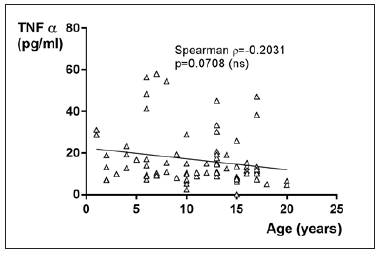
Figure 9:TNF-α in pg/ml at the y-axis (axis of ordinates) and divided into JIAf, Controlf, JIAm and Controlm. The differences in between the groups are statistically not significant (p>0.05).

IL-4
IL-4 in JIA patients shows an average value of 5.2±6.1pg/ ml and is therefore higher than in the controls, which shows an average value of 1.3±1.2pg/ml. With p=0.0182 (Mann Whitney test) the difference between JIA patients and controls is statistically significant (Figure 10). Kruskal Wallis test and Dunn’s posthoc test could not be performed for comparing the subgroups of JIA with the controls, because there are missing values for IL-4 in PS JIA (Figure 11). IL-4 with Spearman ρ=-0.07263 correlates very low, but statistically not significant with the age (p=0.6017).
Figure 10:IL-4 in pg/ml at the y-axis (axis of ordinates) and divided into JIA patients and controls. The difference is statistically significant.

Figure 11:Comparing the subgroups of JIA with the controls could not be performed, because there are missing values for IL-4 in PS JIA.
Regarding gender differences in IL-4 between JIA patients and controls, this groups are divided into JIA Female (JIAf) and JIA Male (JIAm) and Controls Female (Controlf) and Controls Male (Controlm). Levels of IL-4 are on average higher in JIAf than in the other groups. p=0.0053, which is statistically significant, emerges out of the comparison of JIAf with Controlm (p<0.05). The result p>0.05 of comparing the other two groups Controlf and JIAm with each other is statistically not significant (Figure 12). The ROC analysis of IL-4 reveals an area under the curve (AUC) of 0.7744, with a clear distinction of the curve from the line of identity. This is a p=0.0222 statistically significant (Figure 13). The analysis following Youden [32] reveals a cut-off point of IL-4 of >1.68pg/ml to have the highest sensitivity of 76.32 with the highest specificity of 71.43 for distinction of JIA from controls.
Figure 12:IL-4 in pg/ml at the y-axis (axis of ordinates) and divided into JIAf, Controlf, JIAm and Controlm. The differences in between the groups are statistically significant (p<0.05, Kruskal Wallis test, Post hoc test of Dunn).
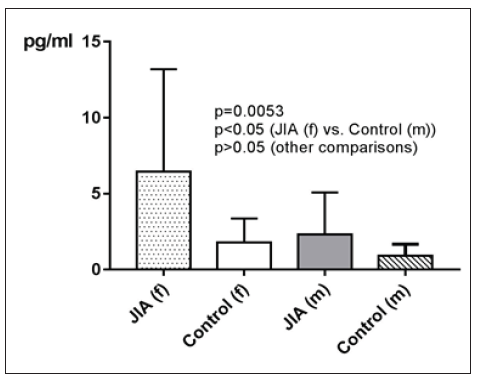
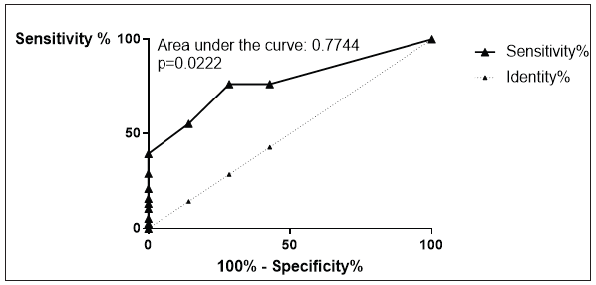
Figure 13:ROC analysis of IL-4. Sensitivity on the ordinate versus 100% - Specificity% on the abscissa. The area under the curve was 0.7744. This was with a p=0.0222 statistically significant.
IL-10
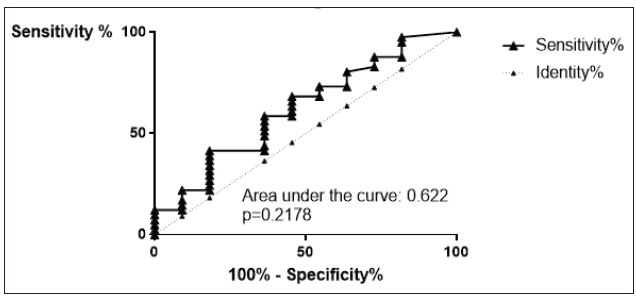
IL-10 in JIA patients shows an average value of 16.7±21pg/ ml and is therefore higher than in the controls, which shows an average value of 9.3±11.3pg/ml. With p=0.2232 (Mann Whitney test) the difference between JIA patients and controls is statistically not significant (Figure 14). Kruskal Wallis test and Dunn’s posthoc test could not be performed for comparing the subgroups of JIA with the controls, because there are missing values for IL-10 in ERA JIA (Figure 15). IL-10 with Spearman ρ=-0.2548 and p=0.0604 correlates slightly inverse, but statistically not significant with the age (Figure 16). However, the p value of 0.06 constitutes a statistical trend towards significance of this effect. Regarding gender differences in IL-10 between JIA patients and controls, this groups are divided into JIA Female (JIAf) and JIA Male (JIAm) and Controls Female (Controlf) and Controls Male (Controlm). With p=0.4191 the differences in between the groups are statistically not significant (Figure 17). The ROC analysis of IL10 revealed an area under the curve of 0.622, with a clear distinction of the curve from the line of identity. However, this was at p=0.2178 statistically not significant (Figure 18).
Figure 14:IL-10 in pg/ml at the y-axis (axis of ordinates) and divided into JIA patients and controls. The difference is statistically not significant.
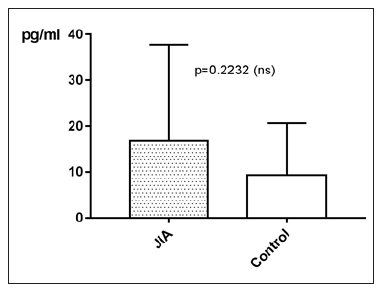
Figure 15:Comparing the subgroups of JIA with the controls could not be performed, because there are missing values for IL-10 in ERA JIA.
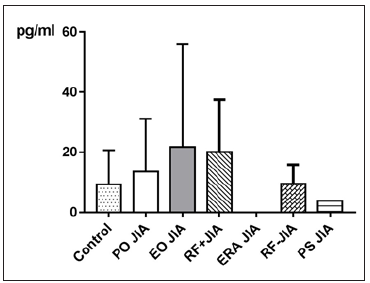
Figure 16:IL-10 in pg/ml at the y-axis (axis of ordinates) with the age in years at the x-axis (x-coordinate). The correlation of IL-10 and the age is statistically not significant (spearman ρ= -0.2548, p=0.0604).
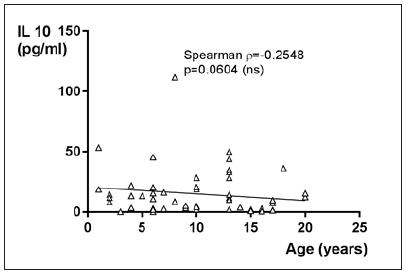
Figure 17:IL-10 in pg/ml at the y-axis (axis of ordinates) and divided into JIAf, Controlf, JIAm and Controlm. The differences in between the groups are statistically not significant (p>0.05, Kruskal Wallis test, Post hoc test of Dunn).
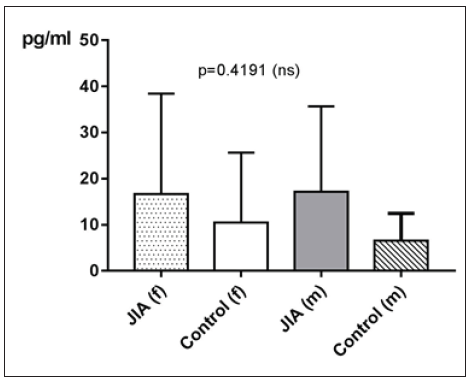
Figure 18:ROC analysis of IL10. Sensitivity on the ordinate versus 100% - Specificity% on the abscissa. The area under the curve was 0.622. This is a p=0.2178 statistically not significant.
IL-17
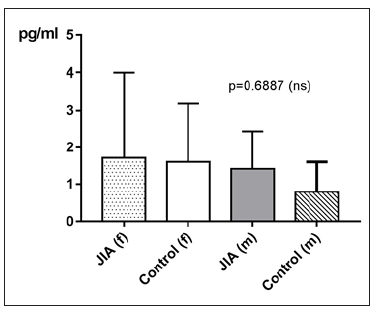
IL-17 in JIA patients shows an average value of 1.6±2pg/ml and is therefore higher than in the controls, which shows an average value of 1.2±1.2pg/ml. With p=0.4800 (Mann Whitney test) the difference between JIA patients and controls is statistically not significant (Figure 19). Kruskal Wallis test and Dunn’s posthoc test could not be performed for comparing the subgroups of JIA with the controls, because there are missing values for IL-17 in ERA JIA (Figure 20). There is a very weak correlation of IL-17 with the age (ρ = 0.1763). However, this effect is with a p value of 0.0037 statistically significant. For examination if there can be found any gender differences in IL-17 between JIA patients and controls, this groups are divided into JIA Female (JIAf) and JIA Male (JIAm) and Controls Female (Controlf) and Controls Male (Controlm). With p=0.6887 the differences in between the groups are statistically not significant (Figure 21). The ROC analysis of IL17 shows a line paralleling the line of identity, which is not significant.
Figure 19:IL-17 in pg/ml at the y-axis (axis of ordinates) and divided into JIA patients and controls. The difference is statistically not significant (Mann Whitney test).
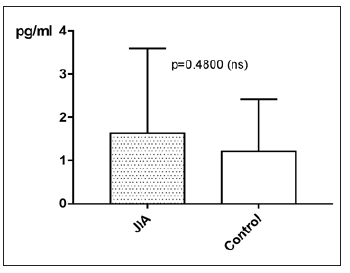
Figure 20:Comparing the subgroups of JIA with the controls could not be performed, because there are missing values for IL-17 in ERA JIA.
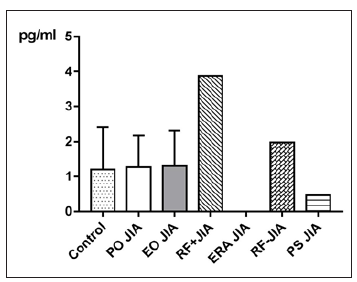
Figure 21:IL-17 in pg/ml at the y-axis (axis of ordinates) and divided into JIAf, Controlf, JIAm and Controlm. The differences in between the groups are statistically not significant (p>0.05, Kruskal Wallis test, Post hoc test of Dunn).
Disease Activity
MCP-1
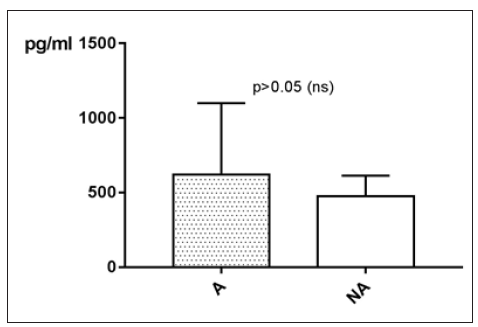
MCP-1 levels taken in an active phase of disease are measured on average higher than MCP-1 levels taken while remission. With p>0.05 (Mann Whitney test) the difference between active phase (A) and remission (NA) is statistically not significant (Figure 22).
Figure 22:MCP-1 in pg/ml at the y-axis (axis of ordinates) and divided into active phase of disease (A) and remission (NA). The difference is statistically not significant.
TNF-α
TNF-α levels taken in an active phase of disease are measured on average lower than TNF-α levels taken while remission. With p>0.05 (Mann Whitney test) the difference between active phase (A) and remission (NA) is statistically not significant (Figure 23).
Figure 23:TNF-α in pg/ml at the y-axis (axis of ordinates) and divided into active phase of disease (A) and remission (NA). The difference is statistically not significant.
IL-4
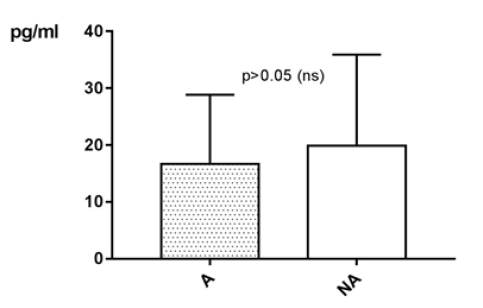
IL-4 levels taken in an active phase of disease are measured on average lower than IL-4 levels taken while remission. With p>0.05 (Mann Whitney test) the difference between active phase (A) and remission (NA) is statistically not significant (Figure 24).
Figure 24:IL-4 in pg/ml at the y-axis (axis of ordinates) and divided into active phase of disease (A) and remission (NA). The difference is statistically not significant.

IL-10
IL-10 levels taken in an active phase of disease are measured on average higher than IL-10 levels taken while remission. With p>0.05 (Mann Whitney test) the difference between active phase (A) and remission (NA) is statistically not significant (Figure 25).
Figure 25:IL-10 in pg/ml at the y-axis (axis of ordinates) and divided into active phase of disease (A) and remission (NA). The difference is statistically not significant.

IL-17
IL-17 levels taken in an active phase of disease are measured on average higher than IL-17 levels taken while remission. With p=0.0249 (Mann Whitney test) the difference between active phase (A) and remission (NA) is statistically significant (Figure 26).
Figure 26:IL-17 in pg/ml at the y-axis (axis of ordinates) and divided into active phase of disease (A) and remission (NA). The difference with p=0.0249 is statistically significant.

Discussion
This study investigated the cytokine profile in a cohort of patients with JIA. Concordant differences in patients with JIA and NHD could be evaluated. The mean values of MCP1, but also the mean values of TNF-α, IL-4, IL-10 and IL-17 are higher in JIA patients than in NHD. The greatest difference has been seen in IL-4, which was at least twice as high in JIA patients as in NHD. Accordingly, to this result, the Area Under the Curve (AUC) in the Reciever Operator Characteristics Analysis (ROC-Analysis) in IL-4 has been 0.7744 with p=0.022, so that this parameter with a cut off of >1.68pg/ml after Youden WJ et al. [32] reaches a sensitivity of 76.32% and a specificity of 71.43% in JIA patients. Regarding the idea that IL-4 might be helpful in the diagnosis of JIA as it is seen in the study of Ziaee V et al. [33] where there were examined polymorphisms in IL-4 in correlation with JIA it could be conceivable that IL-4 could be consulted for diagnosing JIA. The other parameters presented not these differences in patients and NHD.
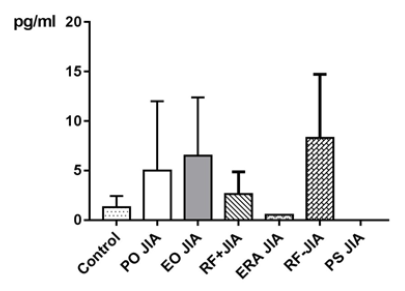
Analysis of the subgroups has shown that values of MCP-1, especially in PO JIA and RF+JIA, compared with the controls have been higher. An elevation of MCP-1 in all subgroups of JIA has already been seen in the study of Yao TC et al. [34]. In TNF-α patients in the subgroup EO JIA have shown substantial higher values compared to NHD. In a study of Date Y et al. [35] where examined polymorphisms in the 5’-flanking promoter/enhancer region of TNF-α and its relationship with JIA. Frequencies of these polymorphisms have been found in systemic JIA, but not in the other subgroups of JIA. IL-4 values have been substantial higher in subgroups PO JIA, EO JIA and RF-JIA compared with NHD. Accordingly, to Ziaee V et al. [33] there is a correlation between polymorphisms in IL-4 and JIA, but no specific subgroups were mentioned. In IL-10 patients of the subgroups EO JIA and RF+JIA have shown higher values compared to NHD. Accordingly, Barnes MG et al. [36] up-regulation of genes associated with IL-10 was prominent in the subgroups PO JIA, RFJIA and systemic JIA.
Also, IL-17 was measured on average higher in JIA patients than in NHD and satisfies its status as pro-inflammatory cytokine [26,27]. The magnitude of the measured values of IL-17 in JIA patients in the present study is comparable with the ones seen in adults with rheumatoid arthritis [37]. Therefore, a treatment with IL-17 inhibitors, especially ixekizumab may be beneficiary, as it was seen in a meta-analysis of studies with patients with rheumatoid arthritis [38] by means of an improvement of the composite response criteria ACR 20 and ACR 50 of the American College of Rheumatology American College of Rheumatology [39,40]. The promising results of the ixekizumab trials in adults raise hope for relief for children suffering from JIA as well. However, ixekizumab is neither approved in Europe, nor in the USA for application in children. Possible side-effects in children are unknown, and it has to be considered, that IL-17A, the target protein of the antibody, has additional functions in childhood, which may be the cause for more severe side effects in children compared to adults. Moreover, the antibody has to be repeatedly injected subcutaneously, a possible problem in younger children. Unfortunately, the subgroups have been too small for a reasonable statistical analysis, which does not mean that the data are not useful in this regard. To the contrary, the looming pronounced differences between the subgroups must, and can be the basis of further for differentiation of subgroups.
For example, IL-4 could be higher at the beginning of an active phase of JIA as later or under therapy, so that standard deviation would increase through this hypothetical effect. At this point we have to take another point into account: If specimens from patients suspected suffering of JIA during a medical appointment are taken, this has to be a short and structured in order, to make sure, that the blood withdrawal is adequate. Also, the further transport and further processing in the laboratory have to be adequate. IL-10 for example is sensitive to heat and the specimen should not even get stored at -20 degrees Celsius, but better at -70 degrees Celsius [41] in order to avoid disintegration of the protein. Disaggregation is much faster by room temperature as it is at -20 degrees Celsius. Therefore, it is highly important that specimens get stocked as fast as possible in a -80 degrees Celsius freezer or in -196 degrees Celsius cold liquid nitrogen. The withdrawal of blood can get better planned in controls and therefore these specimens should be logistically better and faster to handle. It is to expect, that values of IL-10 in JIA patients are normally higher than we measured. The temperature-sensitivity of IL-10 may explain in that case the high standard deviation. Serum samples of IL-4 should get stocked as soon as possible at -20 degrees Celsius for two months or at -70 degrees Celsius when storage for two months to maximum one year [42]. Values of macrophage inflammatory protein-1alpha (MIP-1alpha) and MIP-1beta increase more than twice [43], when blood gets processed later, because both proteins get released out of macrophages after blood withdrawal. In contrast, IL-17, at least IL-17 alpha, appears to be stable even at room temperature [43], and not to be released into the blood after its withdrawal. Therefore, a delay in processing the specimens does not matter, in the case of IL-17; but maybe important in IL-4, for example. Cooling cannot stop but slows down natural temperature-dependent decay process of peptides and of still ongoing decay processes through still in specimen contained proteases or peptidase. Depending on time of taking blood in relation to determination date via immunoassay different ranges of decay have realized. A marker for good data quality is that the magnitude of the standard deviation of the measured values of the single parameters of the JIA group is in the same scale as the magnitude of the standard deviation of the controls.
IL-4 shows the potential to serve as a supporting test when diagnosing JIA, if the diagnosis is already suspected. The relatively high sensitivity and specificity seem to be encouraging, but unfortunately, is not enough for its usage as a screening test. With a sensitivity of 76.32% 20% of the cases will not get diagnosed while the specificity of 71.43% does not give a preferable security of a positive diagnosis: So, there are too few real patients diagnosed with this method and too few of healthy subjects get classified as healthy. A screening should reach, according to more factors like incidence of the disease and importance of the diagnosis, more than 98% specificity and sensitivity [44]. According to Hanova et al. [45] the incidence of children with JIA in the Czech Republic is calculated with 10.8:100.000 in women of 0-15 years and 10.3:100.000 in men of 0-15 years. This is in perfect agreement with the data of the Kaiser Permanente population [46] with a yearly incidence of 16.4 girls and 7.7 boys. Using the determined sensitivity and specificity of IL-4, rather low positive or negative predictive values are the result, making IL-4 unsuitable as a screening test. To reach acceptable predictive characteristics sensitivity and specificity in this case have to be significantly above 99%. This region of magnitude can also not be reached with mathematical combination of the other values of MCP1, TNF-α, IL-10 and IL-17. However, the statistically significant differences in IL-4 between JIA patients and NHD (p=0.0182) confirm earlier results. The suggestion, that several gene polymorphisms at IL-4 and the IL-4 receptor alpha genes found in JIA patients more frequently compared to NHD could promote an autoimmune disease [33] cannot be supported by the present study because this issue was not and could not be examined. Nevertheless, the elevated IL-4 compared to NHD suggests for example the usage of Dupilumab in JIA patients in clinical trials. Dupilumab as an IL-4 receptor antagonist inhibits biological effects of IL-4, because of targeting at the alpha chain of this cytokine. Tests in asthma patients already were promising [47].
Conclusion
Ultimately it can be stated that all parameters in JIA patients systemically show higher values as in NHD. A reason for good data quality is that the magnitude of the standard deviation of the measured values of the single parameters of the JIA group is in the same scale as the magnitude of the standard deviation of NHD. A series of effects could be detected in our research, which awaits confirmation in bigger studies, or even prospective studies with adequate power and enough participants. Especially IL-4 could gain importance for diagnosing JIA. After analyzing the subgroups this seems to be true particularly in PO JIA, EO JIA, RF+JIA and RFJIA patients. Furthermore, it could be worth using this parameter, after serial measurements, for evaluation of disease activity.
References
- Barut K, Adrovic A, Sahin S (2017) Juvenile idiopathic arthritis. Balkan Med J 34(2): 90-101.
- Weiss PF (2012) Diagnosis and treatment of enthesitis-related arthritis. Adolesc Health Med Ther 3: 67-74.
- Oberle EJ Harris JG, Verbsky JW (2014) Polyarticular juvenile idiopathic arthritis-epidemiology and management approaches. Clin Epidemiol 6: 379-393.
- Weiss PF (2017) Polyarticular juvenile idiopathic arthritis: Clinical manifestations, diagnosis, and complications.
- Gaslini G (2016) Juvenile idiopathic arthritis, EULAR Center of Excellence in Rheumatology 2008-2018, University of Genoa Pediatrics II, Rheumatology, IRCCS Institute, Italy.
- Cosickic A, Halilbasic M, Selimovic A, Avdagic H (2017) Uveitis associated with juvenile idiopathic arthritis, our observations. Med Arch 71(1): 52-55.
- Macaubas C, Nguyen K, Milojevic D(2019) Oligoarticular and polarticular JIA: Epidemiology and pathogenesis. Nat Rev Rheumatol 5(11): 616-626.
- Weiss PF (2018) Oligoarticular juvenile idiopathic arthritis.
- Devauchelle-Pensec V, Thepaut M, Pecquery R, Houx L (2016) Managing monoarthritis in children. Joint Bone Spine 83(1): 25-30.
- Tugal-Tutkun I, Quartier P, Bodaghi B (2014) Juvenile idiopathic arthritis-associated uveitis-classification and diagnostic approach. Ocul Immunol Inflamm22(1): 56-63.
- Weiss PF (2013) Evaluation and treatment of enthesitis-related arthritis. Curr Med Lit Rheumatol 32(2): 33-41.
- Chang JH, McCluskey PJ, Wakefield D(2005) Acute anterior uveitis and HLA-B27. Surv Ophthalmol50(4): 364-88.
- Grevich S, Shenoi S(2017) Update on the management of systemic juvenile idiopathic arthritis and role of IL-1 and IL-6 inhibition. Adolesc Health Med Ther 8: 125-135.
- Vastert SJ, Kuis W, Grom AA(2009) Systemic JIA: Developments in the understanding of the pathophysiology and therapy. Best Pract Res Clin Rheumatol 23(5): 655-664.
- Moretti D, Cianchi I, Vannucci G, Cimaz R, Simonini G (2013) Psoriatic juvenile idiopathic arthritis associated with uveitis: A case report. Case Rep Rheumatol 2013: 595890.
- Nigrovic PA (2017) Psoriatic juvenile idiopathic arthritis: Pathogenesis, clinical manifestations, and diagnosis.
- Weselmann K (2017) Psoriatic Arthritis, American college of rheumatology, Atlanta, USA.
- Watts RA, Conaghan PG (2013) Oxford textbook of rheumatology. (4th edn), Oxford University Press, England, UK.
- Giancane G, Consolaro A, Lanni S, Davi S, Schiappapietra B, et al. (2016) Juvenile idiopathic arthritis: Diagnosis and treatment. Rheumatol Ther 3(2): 187-207.
- Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, et al. (2011) Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: Report of forty-six patients from an international multicenter series. Arthritis Rheum 63(2): 545-55.
- Rassow J, Hauser K, Netzker R, Deutzmann R (2016) Dual series biochemistry. (4th edn), Thieme Medical Publishers, New York, USA.
- Heinrich P, Müller M, Graeve L (2014) Löffler/ Petrides biochemistry and pathobiochemistry. (9th edn), Springer, Germany.
- Rus V, Via CS (2007) Systemic lupus erythematosus, Chapter 12, Cytokines in Systemic Lupus Erythematosus. Mosby Publisher, USA, pp. 109-120.
- Mak TW, Saunders ME (2006) The immune response, Chapter 17, Cytokines and Cytokine Receptors, Academic Press, USA, pp. 463-516.
- Iyer SS, Cheng G (2012) Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 32(1): 23-63.
- Zenobia C, Hajishengallis G (2015) Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000 69(1): 142-159.
- Yan JW, Wang YJ, Peng WJ, Tao JH, Wan YN, et al. (2014) Therapeutic potential of interleukin-17 in inflammation and autoimmune diseases. Expert Opin Ther Targets 18(1): 29-41.
- Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte Chemoattractant Protein-1 (MCP-1): An overview. J Interferon Cytokine Res 29(6): 313-326.
- (2018) TNF tumor necrosis factor [Homo sapiens (human)].
- Idriss HT, Naismith JH (2000) TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc Res Tech 50(3): 184-195.
- Gaastra W (1984) Enzyme-Linked Immunosorbant Assay (ELISA). Methods Mol Biol 1: 349-355.
- Youden WJ (1950) Index for rating diagnostic tests. Cancer 3(1): 32-35.
- Ziaee V, Rezaei A, Harsini S, Maddah M, Zoghi S, et al. (2016) Polymorphisms of genes encoding interleukin-4 and its receptor in Iranian patients with juvenile idiopathic arthritis. Clin Rheumatol 35(8): 1943-1948.
- Yao TC, Kuo ML, See LC, Ou LS, Lee WI, et al. (2006) RANTES and monocyte chemoattractant protein 1 as sensitive markers of disease activity in patients with juvenile rheumatoid arthritis: A six-year longitudinal study. Arthritis Rheum 54(8): 2585-2593.
- Date Y, Seki N, Kamizono S, Higuchi T, Hirata T, et al. (1999) Identification of a genetic risk factor for systemic juvenile rheumatoid arthritis in the 5'-flanking region of the TNFalpha gene and HLA genes. Arthritis Rheum 42(12): 2577-2582.
- Barnes MG, Grom AA, Thompson SD, Griffin TA, Pavlidis P, et al. (2009) Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis Rheum 60(7): 2102-2112.
- Elhewala AI, Soliman SG, Labib AA, Mousa WA, Salah D (2015) Interleukin-17 level in rheumatoid arthritis patients and its relation to disease activity. a clinical and ultrasound study. Egypt Rheumatol Rehabil 42: 183-187.
- Kunwar S, Dahal K, Sharma S (2016) Anti-IL-17 therapy in treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatol Int 36(8): 1065-1075.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, et al. (1993) The American college of rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The committee on outcome measures in rheumatoid arthritis clinical trials. Arthritis Rheum 36(6): 729-740.
- American College Committee to Reevaluate Improvement Criteria (2007) A proposed revision to the ACR20: The hybrid measure of American college of rheumatology response. Arthritis Rheum 57(2): 193-202.
- Kenis G, Teunissen C, De Jongh R, Bosmans E, Steinbusch H, et al. (2002) Stability of interleukin 6, soluble interleukin 6 receptor, interleukin 10 and CC16 in human serum. Cytokine 19(5): 228-235.
- (2018) Human interleukin 4(IL-4), Invitrogen Corporation, California, USA.
- Lee JE, Kim JW, Han BG, Shin SY (2016) Impact of whole-blood processing conditions on plasma and serum concentrations of cytokines. Biopreserv Biobank 14(1): 51-55.
- Maxim LD, Niebo R, Utell MJ (2014) Screening tests: A review with examples. Inhal Toxicol 26(13): 811-828.
- Hanova P, Pavelka K, Dostal C, Holcatova I, Pikhart H (2006) Epidemiology of rheumatoid arthritis, juvenile idiopathic arthritis and gout in two regions of the Czech Republic in a descriptive population-based survey in 2002-2003. Clin Exp Rheumatol 24(5): 499-507.
- Harrold LR, Salman C, Shoor S, Curtis JR, Asgari MM, et al. (2013) Incidence and prevalence of juvenile idiopathic arthritis among children in a managed care population, 1996-2009. J Rheumatol 40(7): 1218-1225.
- Sastre J, Dávila I (2018) Dupilumab: A new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 28(3): 139-150.
© 2022 Brunner J. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






