- Submissions

Full Text
Research in Pediatrics & Neonatology
Giant Congenital Melanocytic Naevus: A Case Report and the Potential Risk Factor of Autoimmunity
Hughes NJ*, Verma A and Chetan R
Department of Paediatrics, Southend University Hospital NHS Foundation Trust, UK
*Corresponding author: Hughes NJ, Department of Paediatrics, Southend University Hospital NHS Foundation Trust, UK
Submission: October 26, 2021; Published: December 01, 2021

ISSN: 2577-9200 Volume6 Issue2
Abstract
Background: GCMN is a rare condition associated with neurological abnormalities and risk of
malignant transformation into potentially fatal tumours.
Case presentation: We report a case of profound GCMN in a newborn with a maternal background
of autoimmune disease.
Conclusion: There may be an unexplored shared genetic basis between an abnormal immune milieu
and risk of GCMN. Management of GCMN is multifactorial and patient-centred, with no evidence for
definitive treatment at present.
Keywords: Neonatology; Dermatology; Congenital; Autoimmunity
Abbreviations: GCMN: Giant Congenital Melanocytic Naevus, MAPK: Microtubule-Associated Protein Kinase
Background
GCMN are irregular, hyperpigmented naevi which span over 20cm and may have associated smaller satellite lesions [1]. Histologically, they are junctional, compound or intradermal naevi and often feature hypertrichosis. GCMN have an incidence of less than 1 in 200,000 and are either present at birth or develop from existing melanocytes in the first 2 years of life [2]. They arise largely from gain-of-function somatic mutations in the enzymes BRAF at amino acid V600 (1 in 6 cases) or NRAS at Q6 (4 in 5 cases) [3]. These mutations affect the Microtubule-Associated Protein Kinase (MAPK) signal transduction pathway, leading to abnormal proliferation of embryonic melanoblasts. Consistent with the differentiation of melanocytes from neural crest cells, GCMN are associated with syndromes including type 1 neurofibromatosis [1], lipomatosis [4] and cortical migration abnormalities [5]. Patients with multiple naevi are at greater risk of concurrent neuropathology and are recommended early MR imaging of the brain [6]. GCMN also carry risk of malignant transformation into melanoma and other, rarer neoplasms such as liposarcoma and rhabdomyosarcoma [7]. This is thought to be driven by sporadic mutations or defects in neural crest development and occurs more commonly in larger naevi [2,8].
Case Presentation
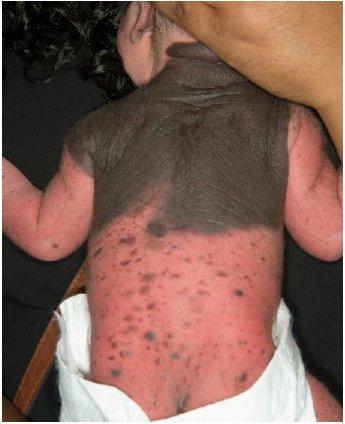
We report a case of GCMN in a newborn with a maternal background of autoimmune disease. His antenatal course was normal, and no physical abnormalities were detected with ultrasound scanning. He was born by uncomplicated vaginal delivery at term, with an extensive GCMN on the upper back (Figure 1) and widespread satellite naevi (Figure 2). The rest of his examination, blood glucose and thyroid function tests were normal. His mother is of Sri Lankan origin, diagnosed with hyperthyroidism, psoriasis and insulindependent type 2 diabetes. His father is of Indian origin and is healthy. Together they have one other son who is well and there is no family history of GCMN.
Figure 1: Large, irregular and hyperpigmented naevus with hypertrichosis.
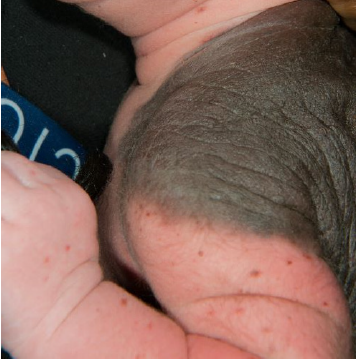
Figure 2: GCMN with widespread satellite lesions.
Discussions and Conclusions
To our knowledge, there have been no reported cases associating
GCMN with autoimmune disease. There is some evidence, however,
that number of melanocytic naevi is related to immunological
disturbance [9,10] such as the abnormal expression of T-cells
and pro-inflammatory cytokines in psoriasis [11]. Furthermore,
immunomodulators which alter the cytokine profile have been
reported to result in eruptions of naevi [12] as well as development
of melanoma [13,14]. It could therefore be hypothesised that
autoimmunity is associated with overstimulation of melanocytes
[15,16] and further studies are required to characterize this
relationship, the potentially underlying genetic cause and whether
control of maternal autoimmune disease has a role in risk reduction
of GCMN.
There are currently no universally accepted guidelines for
treatment of GCMN and the benefit of surgical excision remains
controversial. Recently, the genetic heterogeneity within naevi has
opened the possibility of targeting GCMN with specific inhibitors
[8]. Sporadic mutations in lesions have been found to activate,
for example, the MAPK signaling pathway and MAPK inhibitors
such as trametinib have shown some efficacy [17]. At present,
however, management focuses on sun protection, regular clinical
examination, patient education for early detection of malignant
transformation and minimization of the potential psychosocial
impacts of the condition [18].
To conclude, this is a profound example and personal
perspective of a rare, congenital condition with potential for
malignant transformation. We present a novel hypothesis, with
evidence from this case and the wider literature of a predisposition
to GCMN in those with genetic mutations also present in
autoimmune disease. Further research is required to exclude solely
a fortuitous concurrence between GCMN and what are relative
common diseases, as well as to develop more effective, targeted
treatments.
Patient’s Perspective
I granted consent for publication of my baby’s photographs to increase the information available about GCMN and its potential complications. When he was born, my husband and I were not expecting him to have any problems and we were frightened by the lesion. Neither we nor the midwife present had ever seen or heard of GCMN. I was worried that my background of psoriasis had a role in causing the condition and so I am pleased that his case may encourage research into whether any risk is conveyed by maternal autoimmune disease. Most of all, I hope that this report will help my son to better understand his condition, allow him to take pride and ownership over his unique GCMN and in doing so overcome any psychosocial impacts it may have on his future.
References
- Weedon D, Strutton G, Rubin AI, Weedon D (2010) Weedon’s skin pathology. Churchill Livingstone, Elsevier, London, UK, pp. 1041.
- Yun SJ, Kwon OS, Han JH, Kweon SS, Lee MW, et al. (2012) Clinical characteristics and risk of melanoma development from giant congenital melanocytic naevi in Korea: A nationwide retrospective study. Br J Dermatol 166(1):115-123.
- Etchevers HC (2014) Hiding in plain sight: Molecular genetics applied to giant congenital melanocytic nevi. J Invest Dermatol 134(4): 879-882.
- Kumar I, Aggarwal P, Rai T, Gupta V (2019) Posterior quadrantic dysplasia with localized hemimegalencephaly in a patient with giant congenital melanocytic nevus: First case report. Neuroradiol J 32(3): 210-214.
- Agarwal A, Dhameja N, Kar G (2019) Giant congenital melanocytic nevus associated with lipoma in an Indian man. BMJ Case Rep 12(7): e228688.
- Waelchli R, Aylett SE, Atherton D, Thompson DJ, Chong WK, et al. (2015) Classification of neurological abnormalities in children with congenital melanocytic naevus syndrome identifies magnetic resonance imaging as the best predictor of clinical outcome. Br J Dermatol 173(3):739-750.
- Pitjadi TM, Wadee R, Grayson W (2019) Rhabdomyosarcoma arising in a giant congenital melanocytic naevus: Case report and literature review. Dermatopathology(Basel) 6(2): 91-98.
- Belysheva TS, Vishnevskaya YV, Nasedkina TV, Emelyanova MA, Abramov IS, et al. (2019) Melanoma arising in a giant congenital melanocytic nevus: Two case reports. Diagn Pathol 14(1):21.
- Balato N, Di Costanzo L, Balato A, Patruno C, Scalvenzi M, et al. (2011) Psoriasis and melanocytic naevi: Does the first confer a protective role against melanocyte progression to naevi? Br J Dermatol 164(6): 1262-1270.
- Di Cesare A, Riitano A, Suppa M, Fidanza R, Zangrilli A, et al. (2013) Frequency of melanocytic nevi in psoriatic patients is related to treatment and not to disease severity. J Am Acad Dermatol 69(6): 947-953.
- Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, et al. (2016) Psoriasis. Nat Rev Dis Prim 2(1):16082.
- Bovenschen HJ, Tjioe M, Vermaat H, de Hoop D, Witteman BMJ, et al. (2006) Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol 154(5):880-884.
- Finet A, Amini-Adle M, Balme B, Colson F, Thomas L (2013) Nodular progression of lentigo malignant melanoma during a treatment with tocilizumab: Cause or coincidence? Clin Rheumatol 32(2): 277-280.
- Bonny M, Buyse V, Suys E (2012) Rapidly progressive malignant melanoma in a patient treated with tocilizumab. J Am Acad Dermatol 67(2): e78-79.
- Elder JT (2017) The quest for psoriasis autoantigens: Genetics meets immunology in the melanocyte. J Invest Dermatol 137(10): 2042-2045.
- Billi AC, Gudjonsson JE, Voorhees JJ (2019) Psoriasis: Past, present, and future. J Invest Dermatol 139(11): e133-142.
- Mir A, Agim NG, Kane AA, Josephs SC, Park JY, et al. (2019) Giant congenital melanocytic nevus treated with trametinib. Pediatrics 143(3): e20182469.
- British Association of Dermatologists (2017) Melanocytic naevi (pigmented moles). Br Assoc Dermatologists 258474: 1-5.
© 2021 Hughes NJ. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






