- Submissions

Full Text
Research in Pediatrics & Neonatology
Human Para-echovirus in Newborn: From the Case Report to the Review of the Disease
Luciana Romaniello, Sergio Manieri*, Maria P Mirauda, Rosa Lapolla, Raffaele Pecoraro and Elisabetta Placella
Department of Pediatrics, San Carlo Hospital, Potenza, Italy
*Corresponding author: Sergio Manieri, Department of Pediatrics, San Carlo Hospital, Potenza, Italy
Submission: June 25, 2021; Published: July 09, 2021

ISSN: 2577-9200 Volume6 Issue1
Abstract
Human Par-echovirus (HPeV) is a potentially severe viral infection in neonates and young infants. This virus often causes gastrointestinal or respiratory illness in young children and, infrequently, causes disease in older children and adults. In infants can present with a sepsis-like picture, often with Central Nervous System (CNS) involvement, which is difficult to differentiate clinically from bacterial sepsis. We describe a case of parechovirus infection in an 8-day-old female newborn with a very severe clinical picture and serious CNS involvement. Starting from this case report we provide an overview of HPeV infections which, in our opinion, are currently underdiagnosed while should be considered more carefully.
Keywords: Human para-echovirus; CNS involvement; Neonates and young infants
Introduction
Human Par-echovirus (HPeV) is a very new genus classified under the Picornaviridae family that is characterized as small, non-enveloped and uniquely stranded positive-sense RNA virus, that primarily infects infants and young children but infrequently causes disease in older children and adults. HPeVs are closely related to enteroviruses (named Echoviruses 22 and 23) that cause diarrhea, colds and other infections. Parechovirus is of two types, Parechovirus A and Parechovirus B, and is currently divided into 19 genotypes. Their initial description as Echovirus 22 and 23, further serotypes of human Enteroviruses, was based on similarities in their original clinical presentations, their enterovirus-like cytopathology on virus isolation and inferred small size based on ultrafiltration [1].
This kind of infection in infants can manifest itself with a sepsis-like picture, often with Central Nervous System (CNS) involvement, which is difficult to differentiate clinically from bacterial sepsis. Molecular diagnostic methods are essential for early diagnosis, mostly in children younger than 6 months of age with characteristic presentations without another confirmed diagnosis including febrile illnesses with other suggestive features (eg, rash, seizures), sepsis syndromes (including shock), and suspected meningoencephalitis, which may be detected by Magnetic Resonance Imaging (MRI) only. There are no effective antiviral therapies. Treatment is primarily supportive, including management of complications. Therefore, early diagnosis is important to reduce the use of antibiotics and shorten the length of hospitalization in the case of minor illness [2]. Follow-up by a pediatrician is recommended [3].
Case Report
A healthy female infant, born by vaginal delivery in July 2018 at 40 weeks of gestation, presented at 8 days old to the emergency room of San Carlo Hospital in Potenza, for a 1 day history of hyporeactivity, reduced oral intake, fever and tachypnea. The newborn was highly distressed and inconsolable, and had been persistently crying, with poor breastfeeding and a low-grade fever (37.5 °C) since the previous day. There had been no contact with sick people.
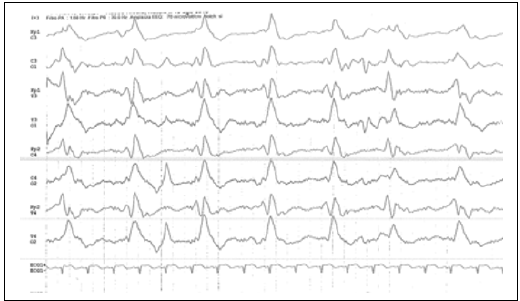
Her vital signs were: body temperature of 39 °C, respiratory rate of 65 breaths/min and heart rate of 170 b/min. The initial physical examination were otherwise normal, without any respiratory or gastrointestinal symptoms and signs, no neurologic or cranial nerve deficits. There was a pale erythematous rash on the limbs and the trunk. At admission, routine laboratory tests were normal. Laboratory tests on admission revealed a slight increase in C-Reactive Protein (CRP) level (10 mg/l), mild leukopenia with marked relative monocytosis (white blood cell count 4.850/ul: monocytes 20%, neutrophils 30%, lymphocytes 40%), normal hemoglobin level, and normal platelet count. Chest radiograph was normal. The neonate was admitted to the Neonatal Intensive Care Unit (NICU) with a diagnosis of suspected infection, intravenous antimicrobial therapy (ampicillin and netilmicin) and Intravenous acyclovir (according to Unit protocol) was started. Twelve hours after her admission, the baby had a convulsive episode characterized by myoclonic seizures and staring. Anticonvulsant therapy (phenobarbital plus phenytoin) was started, but the baby continued to have seizures requiring control by midazolam. The Electroencephalogram (EEG) performed before and after Phenytoin infusion and Midazolam administration was compatible with a status epilepticus (Figure 1), gradually regressed with the therapy.
Figure 1: Brain RMI.
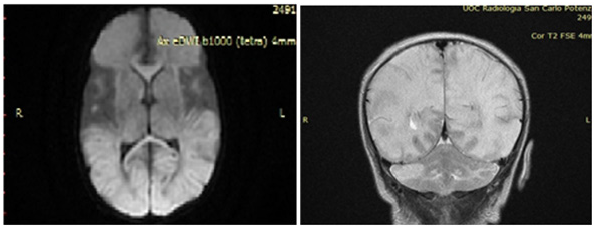
Figure 2:EEG.
Bacterial cultures of specimens collected at the time of admission were negative and also viral investigations on blood, urine, nasopharyngeal swab and stool samples resulted negative for all investigated pathogens (Enteroviruses, HSV1, HSV2, cytomegalovirus, Epstein-Barr virus, human herpesvirus 6, parvovirus B19, adenovirus). A lumbar puncture was also performed, and CSF tested positive for HPeV (by Film Array, but without the possibility of identifying the specific genotype). At that point, acyclovir was stopped and was started Intravenous Immunoglobulin (IVIG) 1gr/kg for 3 days. After 5 days of hospitalization she performs brain MRI (Figure 2), that showed cerebral White Matter (WM) suffering, extended into the subcortical periventricular WM and in the deep WM of both cerebral hemispheres, particularly in the left anterior frontal region and in the right parietal region, with focal thinning of the cortex. Areas with similar characteristics of signal intensity are documented in the cerebellar hemispheric site bilaterally, to a greater extent on the right. The descriptive findings appear to refer, in the first hypothesis, to areas of tissue suffering on a vasculopathic basis, probably secondary to the known basic infectious picture. At discharge the Neuropsychiatric evaluation showed: spontaneous motility characterized by a poor repertoire, with some aspects of excessive synchronization, posture influenced by persistence of asymmetrical tonic reflex in the neck, poor control of the head and ocular motor skills. The last EEG traces continued to show excess of discontinuities and plurifocal paroxysmal anomalies. The patient gradually improved and she was discharged on day 27, maintenance therapy with phenobarbital was recommended. She was inserted into a clinical and instrumental follow-up program to define her long-term prognosis. At the control, after one month MRI showed cystic encephalomalacic evolution of the known lesional areas reported at the previous examination. Twelve months later, the child presented microcephaly ([head circumference was 35cm at birth (50-75th percentile), 37cm at 3 month (< 3rd percentile), and 43cm at 12 months (<3rd percentile)] and a psychomotor impairment. It is well know that the neurodevelopmental outcomes of HPeV meningoencephalitis are variable, ranging from cerebral palsy to epilepsy to normal development, which are well-correlated with the severity of brain MRI and with therapy-resistant seizures. Our case had significant neurological sequelae and the WM involvement may explain this kind outcome. This evidence, in our opinion, might suggest the prognostic implication of MRI findings.
Discussion
Human par-echoviruses were formerly part of the echovirus genus in the 1960s but were assigned their own genus in the 1990s [4]. Currently, HPeVs are classified in the genus Parechovirus, which is divided into two species, Parechovirus A and Parechovirus B. Parechovirus A is subdivided into 19 genotypes, HPeV-1 to-19, based on phylogenetic analysis of VP1 sequences (a polyphytide chain named VP1), while Par-echovirus B comprises Ljungan viruses 1 to 4 [2]. HPeVs have a single-stranded RNA genome of approximately 7300 bases in length, nearly all of which have a direct protein-coding function. The HPeV capsid consists of 60 protomers formed by three non-identical polypeptide chains (VP0, VP1 and VP3).
HPeVs have atypical biological and molecular properties, such as unusual cytopathic actions and the lack of cleavage of the VP0 protein into VP4 and VP2 (this last unusual characteristic has been reported also for Kobuvirus), which leads to a virion particle with only three rather than four capsid proteins [4]. Of the all genotypes, HPeV genotypes 1, 3 and 6 are most commonly associated with human disease. In European studies HPeV was detected in 3-8% of children presenting to emergency with undifferentiated fever and appears to be more common in younger children and in children requiring hospital admission, where up to 15-20% of children with fever without source are subtype by the age of 2 years. More serious disease has been associated with HPeV3 infection, which was first associated with acute flaccid paralysis in a young HPeV-positive [5]. Serological data from Europe and Japan show that 90% of infants have been infected with at least one HPeV [2]. HPeV1 is the most common Para-echovirus in Europe, followed by HPeV3. HPeV1 infections typically occur at higher frequencies in late summer and early winter months, similar to the seasonal pattern of human enteroviruses. Instead, HPeV3 has been isolated most frequently in summer months. HPeV3 is further unusual in exhibiting a biannual cycle of infections, in Europe occurring almost exclusively in even numbered years since 1988 [1]. A very important aspect concerns the clinical presentation and complications of HPeV infection. It is now known that the primary site of HPeV replication is the respiratory and gastrointestinal tract; intestinal replication leads to the rapid spread of infectious virus in faeces readily detectable by virus isolation and RT-PCR methods. In addition to faecal/ oral transmission, infections may also occur through respiratory routes, with infection and virus shedding detectable in respiratory secretions. Furthermore, virus can spread via the blood stream to other organs causing systemic illness [6].
HPeV infections in the first years of life are characterized by a wide variety of clinical manifestations, ranging from asymptomatic to very severe infections, including sepsis, meningitis and encephalitis, which mainly occur in neonates and infants under the age of 3 months. Like enteroviruses, the epidemiology and clinical syndromes associated with HPeV vary by genotype [2]. HPeV1 and HPeV6 most affect infants > 6 months of age and are associated with milder gastrointestinal symptoms. HPeV may be isolated from respiratory specimens in children presenting with influenzalike illness and in faecal specimens from children presenting with viral diarrhoea [7]. A number of case reports and small studies propose associations of HPeV with a wide range of other diseases, including lymphadenitis (HPeV4), myositis (HPeV3), hemolytic uremic syndrome (HPeV1), myocarditis (HPeV1), TORCH Syndome (HPeV4) and Necrotising enterocolitis (HPeV1). Reye’s syndrome, which is characterized as an acute, non-inflammatory encephalopathy with hepatic dysfunction and fatty infiltration, has been similarly associated with lethal HPeV5 and HPeV6 infections. Further studies, with improved diagnostic assays, are required to determine the frequencies of these disease associations relative to other viral and non-viral aetiologies [1].
A maculopapular or erythematous rash is a common sign of HPeV infection, but there is little information concerning the frequency and characteristics of the rash during the course of the disease. A study concluded that rash limited to the distal extremities (particularly the palms of the hands and soles of the feet, a rare physical finding in neonates and young infants) may be considered a diagnostic clue of HPeV3 infection [8]. In particular, when the presence of HPeV in the community is known, clinical forms of “hot, red, angry babies” with features of sepsis should prompt clinicians to consider HPeV infection [2]. Indeed, during an HPeV-3 outbreak in Australia, the recognition of this triad of fever, rash, and severe irritability made it possible to quickly diagnose infected infants [9]. The majority of severe HPeV infections are caused by HPeV-3 (Figure 3) and present in infants 3 months of age as sepsis, sepsis-like illness, or CNS infection. It is not known why HPeV3 is specifically associated with severe neonatal infections, but the underlying basis may be at least partly epidemiological. The increased severity of the disease in infants with HPeV3 infection is explained by the fact that the frequency of previous HPeV3 infections may be lower than that of other HPeV types. As a consequence, neonates and young infants would not be protected as frequently by maternal antibody after birth as they would be from HPeV1 and possibly other types [6].
Figure 3:Human Paraechovirus 3.
Seizures are a common presentation of HPeV infections of the CNS, with one study describing seizures in 90% of HPeV-infected infants with CNS involvement [2,10]. Currently the focus is on the development of white matter damage in young children. HPeV white matter lesions are indistinguishable from those reported for enterovirus infections and hypoxic-ischemic encephalopathy is variable, from case with diffuse signal intensity changes and punctate white matter lesions, to cysts within the WM. Unlike periventricular leukomalacia, which is more frequent in pre-term infants, the cerebral WM abnormalities extend into the subcortica areas and involve entire tracts of fibers. The injured WM may be due to microglia activation as a result of the activation of intracellular toll-like receptors (TLR 7 and TLR 8), which are involved in the immune response to HPeV. Microglial activation would lead to the release of reactive oxygen and nitrogen species, as well as pro-inflammatory cytokines that are toxic for pre-myelinating oligodendrocytes and axon development [9,11]. The neurological sequelae, which are present in a minority of cases, include cerebral palsy, learning disability, epilepsy and suspected developmental abnormalities [9,10]. Apart from meningoencephalitis, HPeV has also been reported to cause acute flaccid paralysis [2,12]. HPeV also seems to be related to the development of Acute Disseminated Encephalomyelitis (ADEM), which has been described in at least two separate cases [2,13].
Regarding laboratory diagnostics, infection parameters in blood are often within a normal range. Some patients with severe HPev infections develop thrombocytopenia and reports of elevated levels of Aspartate Aminotransferase (ASAT) and Lactate Dehydrogenase (LDH) vary from a low proportion to 100% of patients [2]. A lack of CSF pleocytosis and normal protein and glucose levels in CSF are common in patients with HPeV CNS infections [14,15]. This phenomenon of uninflamed CSF was recognized previously in enteroviral CNS infections [16]. In general, the diagnostic tools available for HPeV infection are not numerous. Unfortunately, routine diagnostic tests for diagnosis are rarely available in clinical diagnostic laboratories, despite current evidence of a significant role for HPEV in several serious diseases, such as neonatal sepsis. Inevitably, the actual involvement of HPeV in clinical illnesses is substantially underestimated as result. Viral culture has been used for the initial diagnosis of HPeV infections, but propagation in viral culture media has proven difficult. Serological assays have been developed for research but none are commercially available for diagnostic purposes [2]. Currently, the availability of molecular approaches with RT-PCR and genotyping has increased the possibilities for diagnostics. Conventional methods, such as viral culture and immunofluorescence assay, together with molecular methods facilitate comprehensive viral diagnosis [2]. Viral cultures were initially used to diagnose HPeV infections, but propagation proved difficult. In particular, HPeV3 grows less efficiently than HPeV1 and HPeV2, and only in a limited number of cell lines. Cell culture models currently exist that are used in high-throughput screening of antiviral compounds and serological tests have been developed for research purposes but are not commercially available. The most appropriate diagnostic method is using reverse transcriptase real-time polymerase chain reaction (RT-PCR), which is more sensitive and specific in detecting RNA in different body specimens, easier and more rapid to perform than viral cultures [8]. As mentioned, HPeVs can be detected on blood and CSF samples, in respiratory secretions and in stool samples. Quantitative RT-PCR in feces has led to the highest detection rate in symptomatic pediatric patients and fecal HPeV shedding can occur up to two months after the onset of symptoms [8]. In HPeV3 infections with an HPeV-3 sepsis-like pattern, the virus can be detected in the serum immediately, as early as the day of onset of symptoms. Serum viral loads were highest upon admission to the hospital and decreased rapidly in the following days [2].
Regarding the therapeutic approach, there are no currently FDA-approved therapies available for HPeV infections in any age group. However, the two categories of treatment that are considered most frequently are IVIG, which may provide neutralizing antibody for clearance of virus, as well as experimental antiviral therapies that inhibit enterovirus replication [17]. IVIG has been utilized in many centers for treatment of neonates with severe enterovirus and parechovirus sepsis, although its use is predominantly based on anecdotal experience rather than randomized controlled clinical trial. In general, IVIG have been used as supportive therapy in the case of severe HPeV infection, but randomized studies are needed to prove their therapeutic efficacy [2]. An extensive review of the compounds that can inhibit picornavirus replication has recently been published, but it did not include any data concerning compounds that can potentially inhibit HPeVs [18,19]. The drugs under study for anti-HpeV therapy are capsid and 3C-protease inhibitors. In particular, drugs with capsid inhibitory properties appear to hold great promise in treating picornavirus infections. Of these, pleconaril has been evaluated most extensively in clinical trials [18,2]. Another option would be the use of monoclonal antibodies. The protective antibodies against human enteroviruses are presumably neutralizing type-specific against the VP1 capsid protein and will not have the broad cross-neutralizing capacity as recently described in the case of influenza virus. However, this approach might be feasible for the much smaller HPeV group, and neutralizing antibodies against VP0 have shown cross-reactivity [2,20]. The role of corticosteroids remains unknown.
Conclusion
In light of this evidence we believe that in general HPeV infections are underdiagnosed, while they should be considered, together with enteroviruses, in the clinical and diagnostic evaluation of severe neonatal diseases. However, MRI changes are not very specific in HPeV encephalitis. Similar changes have been described in neonatal enterovirus encephalitis, where, interestingly, an absence of CSF pleocytosis often occurs. Furthermore, these imaging findings are reminiscent of perinatal WM injury from other causes that are known to result in periventricular leukomalacia and Hight risk of the cerebral palsy. The periventricular and subcortical WM is vulnerable in young children, especially premature infants, and similar patterns of cellular damage can be initiated by ischemia, inflammation or both [21]. There is the need for longer-term follow-up of the broad spectrum of HPeV CNS disease, stratified by genotype where it is known, to definitively determine the connection between HPeV CNS infection and long-term neurologic outcome. For all these reasons HPeVs should be born in mind, especially in the case of very young patients with sepsis, meningitis or encephalitis; the detection of HPeV should therefore be incorporated in routine diagnostic panels for viral infections.
References
- Harvala H, Simmonds P (2009) Human par-echoviruses: Biology, epidemiology and clinical significance. J Clin Virol 45(1): 1-9.
- Olijve L, Jennings L, Walls T (2017) Human parechovirus: An increasingly recognized cause of sepsis-like illness in young infants. Clinical Microbiol Rev 31(1): 1-17.
- Britton PN, Jones CA, Macartney K, Cheng AC (2018) Parechovirus: An important emerging infection in young infants Med J Aust 208(8):365-369.
- Hyypia T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, et al. (1992) A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci USA 89: 8847-8851.
- Stanway G, Joki Korpela P, Hyypiä T (2000) Human parechoviruses--biology and clinical significance. Rev Med Virol 10: 57-69.
- Harvala H, Wolthers KC, Simmonds P (2010) Parechoviruses in children: Understanding a new infection. Curr Opin Infect Dis 23(3): 224-230.
- Bergallo M, Galliano I, Montanari P, Brusin MR, Gabiano C, et al. (2016) Molecular detection of human parechovirus in under-five-year-old children with gastroenteritis. J Clin Virol 85: 17-21.
- Esposito S, Rahamat-Langendoen J, Ascolese B, Senatore L, Castellazzi L, et al. (2014) Pediatric parechovirus infections. J Clin Virol 60(2): 84-89.
- Khatami A, McMullan BJ, Webber M, Stewart P, Francis S, et al. (2015) Sepsis-like disease in infants due to human parechovirus type 3 during an outbreak in Australia. Clin Infect Dis 60(2): 228-236.
- Verboon-Maciolek MA, Groenendaal F, Hahn CD, Hellmann J, van Loon AM, et al. (2008) Human parechovirus causes encephalitis with white matter injury in neonates. Ann Neurol 64(3): 266-273.
- VolpeJJ (2008) Neonatal encephalitis and white matter injury: More than just inflammation? Ann Neurol 64: 232-236.
- Alam MM, Khurshid A, Shaukat S, Sharif S, Rana MS, et al. (2012) Identification of human parechovirus genotype, HPeV-12, in a paralytic child with diarrhea. J Clin Virol 55(4): 339-342.
- Obermeier PE, Karsch K, Hoppe C, Seeber L, Schneider J, et al. (2016) Acute disseminated encephalomyelitis after human parechovirus infection. Pediatr Infect Dis J 35(1): 35-38.
- Schuffenecker I, Javouhey E, Gillet Y, Kugener B, Billaud G, et al. (2012) Human parechovirus infections, Lyon, France, 2008- 10: evidence for severe cases. J Clin Virol 54(4): 337-341.
- Harvala H, Griffiths M, Solomon T, Simmonds P (2014) Distinct systemic and central nervous system disease patterns in enterovirus and parechovirus infected children. J Infect 69(1): 69-74.
- Verboon-Maciolek MA, Groenendaal F, Cowan F, Govaert P, Van Loon AM, et al. (2006) White matter damage in neonatal enterovirus meningoencephalitis. Neurology 66(8): 1267-1269.
- Harik N, DeBiasi RL (2018) Neonatal nonpolio enterovirus and parechovirus infections. Semin Perinatol 42(3): 191-197.
- Wildenbeest JG, Harvala H, Pajkrt D, Wolthers KC (2010) The need for treatment against human par-echoviruses: How, why and when. Expert Rev Anti Infect Ther 8(12): 1417-1429.
- De Palma AM, Pürstinger G, Wimmer E, Patick AK, Andries K, et al. (2008) Potential use of antiviral agents in polio eradication. Emerg Infect Dis 14(4): 545-551.
- Joki-Korpela P, Roivainen M, Lankinen H, Pöyry T, Hyypiä T (2000) Antigenic properties of human parechovirus 1. J Gen Virol 81(Pt 7): 1709-1718.
- Britton PN, Dale RC, Nissen MD, Crawford N, Elliot E, et al. (2016) Parechovirus encephalitis and neurodevelopmental outcomes. Pediatrics 137(2): e20152848.
© 2021 Sergio Manieri. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






