- Submissions

Full Text
Research in Pediatrics & Neonatology
Adrenal Cortex Carcinoma: One of the Rarest Retroperitoneal Tumors in children
D Hanine1,2*, B Rouijel1,2, A El Baoudi1,2 and M Kisra1,2
1Pediatric Surgery Department “A”, Children’s Hospital of Rabat, Morocco
2Faculty of Medicine and Pharmacy, Mohammed V University Rabat, Morocco
*Corresponding author:D Hanine, Pediatric Surgery Department “A”, Children’s Hospital of Rabat, Faculty of Medicine and Pharmacy, Mohammed V University Rabat, Morocco
Submission: April 01, 2021 Published: April 29, 2021

ISSN: 2577-9200 Volume5 Issue4
Abstract
Adrenal cortex is a cancer of the adrenal cortex, rare in children; it accounts for 0.2% of pediatric cancers with a ratio of 1.5 in girls and is sometimes part of predisposing syndromes. The clinical signs suggestive of adrenocortical carcinoma are most frequently endocrine, present in 90% of cases. The hormonal increased secretion is made up of cortisol, androgens, estrogen and more rarely, Aldosterone. Symptoms of virilization are most commonly seen and may or may not be associated with Cushing’s syndrome. Arterial hypertension (hypertension) caused by increased secretion of aldosterone is rarer. More rarely, the mode of discovery is the presence of an abdominal mass, most often associated with abdominal pain, or sometimes local signs of compression.
The prognosis of these tumors, as well as the survival rate, is very variable depending on the tumor stage. It is very pejorative for stage III and IV tumors, compared to stage I and II tumors. The 5-year overall survival rate, all stages combined, fluctuates between 49% and 57% depending on the series, but it can vary between 20 and 90% depending on the tumor stages. Surgery is the cornerstone of treatment, hence the question all surgeons ask themselves: the possibility of complete excision. We report the case of an infant, brought to consult for a Genu Varum, who’s clinical and radiological investigations concluded in an adrenal cortex. The treatment was surgical with an anatomopathological study confirming the diagnosis. The outcome was favorable thereafter with regression of endocrine signs over a 6-month follow-up. At the end of this observation, we highlight this very rare pathology as well as its management.
Keywords: Adrenocortical carcinoma; Virilizing tumors; Adrenal tumors; Child
Introduction
Adrenocortical Carcinoma (ACC) are rare tumors that have a bimodal distribution, the first peak is in children less than five years and the second around the fifth decade [1]. Although most adult ACC are non-functional, in the pediatric age group, nearly 95% are functional [2]. Virilization is the most common abnormality and Cushing’s syndrome and hyperaldosteronism are less frequent [3]. We begin the review with a clinical case of a 16-month-old infant, and then we will discuss this very rare pathology as well as its management.
Materials & Methods
Our study was done in March 2021 at the pediatric surgery department “A” at the
Children’s Hospital of Rabat, the case studied is a 16-month-old boy brought by his parents for
hirsutism and genu varum. Without history, the child had since the first months a pubic hair.
On examination, his weight was 12kg, height was 85cm and blood pressure was 90/60mmHg.
He had no cushingoid face, no moon face, not stretch marks, but pubic and body hair well
developed and gynecomastia. The external genitalia were male with normal sized testicles
but an increased penis of 6cm in size. On the osteoarticular level, the articular amplitudes
were normal. The clinical examination showed a harmonious bilateral genu varum. There
were no neurocutaneous markers. There was no family history of malignancy. Laboratory
investigations revealed normal hematological and biochemical parameters. Morning (0800
hours) serum cortisol was 2.9μg / Dl. Other hormonal levels were normal.
Ultrasound of abdomen revealed a tissue mass at the level of the superior pole of the right
kidney, heterogeneous hypoechoic, of regular contours, exerting a mass effect on the renal
cortex without separating border; measuring 52x51x44mm. Computed Tomography (CT) of the abdomen showed a right retroperitoneal mass, well-defined
tissue, of regular contours, without calcifications, presenting a
heterogeneous enhancement after injection of the contrast product;
measuring 51x43x49mm (Figure 1). The extension assessment was
negative. We decide to do a first surgery. Surgical exploration found
a fully encapsulated right adrenal mass. This mass pushed back the
inferior vena cava and the renal pedicle without infiltrating them
with a separating border. The dissection of the mass above the liver
was easy. We continued dissection down below the Kidney (Figure
2). We did a complete resection of the encapsulate mass (Figure
3). There was no locoregional ganglion. The anatomopathological
study concluded in an Adrenocortical Carcinoma (ACC). The
postoperative follow-up was simple with regression of signs of
virilization in the postoperative months.
Figure 1:Abdominal CT showing the tumor.
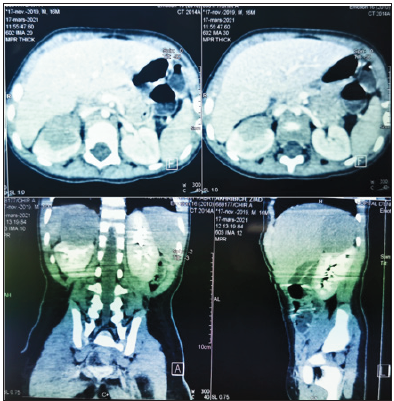
Figure 2:Surgical exploration showing the mass at the upper pole of the Kidney.
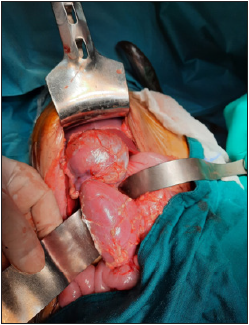
Figure 3: The fully encapsulated resected mass.

Discussion
Adrenal cortex in children is rare, accounting for only 0.2% of pediatric cancers. The incidence of ACC follows a bimodal distribution with a peak during the first ten years of life and particularly before four years. It is estimated at 0.4/million per year for the first four years of life [4]. The ACC can be on the right or left side, however some authors recognize a greater frequency of these tumors on the left side. Bilateral tumors have been reported in 2-10% of cases [5]. The sex ratio changes with the age at the time of diagnosis of the disease. There is an overall female predominance, accentuating among those under three (ratio: 1.7/1) and especially among those over 13 (ratio: 6.2/1) [2]. ACCs can occur as part of specific predisposing syndromes that are currently well identified, the two main ones being: [6].
Li Fraumeni syndrome
Li Fraumeni syndrome is an autosomal disease characterized by a predisposition and early onset of several cancers; this syndrome should be looked for systematically in children with ACC.
Beckwith-wiedemann syndrome
Beckwith-Wiedemann Syndrome is characterized by visceralomegaly, hyperglyceridemia, malformations, and predisposition to malignant tumors.
Other predisposing syndromes
A. NEM1: multiple endocrine neoplasia 1: rarely involved in
children.
B. Carney complex: mainly responsible for lumpy disease of
the adrenal cortex.
C. Congenital adrenal hyperplasia most often responsible
for adenomas.
In children, the majority of ACCs are of the secreting type while only less than 50% of these tumors appear functional in adults. In more than 90% of cases the clinical signs suggestive of ACC are most frequently endocrine, most often made of a syndrome of virilization associated or not with Cushing syndrome, feminization syndrome or Conn syndrome are rare [7].
Virilizing tumors are the most frequent presentation of the childhood ACC, it largely predominates in the series studied: 95% for SABBAGA [8], 76% for C TEINTURIER [9], 66% for Sprague RG [5], 55% for Sandrini R [7]. These tumors are manifested by iso-sexual precocious pseudo-puberty in boys and heterosexual in girls with a picture of virilization. Most often the first sign that attracts attention is an enlarged clitoris in girls or the penis in boys. Sometimes attention is drawn to the appearance of pubic hair, accelerated growth, change in voice [7].
In boys: The picture is that of precocious pseudo-puberty with hypertrophy of the penis which is constant, the length of the penis is variable ranging from 4 to 10cm, our patient had a penis of 5cm; the testicular volume remains normal for the age[10]. This points to adrenal pathology, the presence of pubic hair is constant in both sexes to varying degrees, it is not proportional to the degree of hypertrophy of the clitoris or the penis [11]. Facial and axillary hairs are also reported in some observations.
Regarding the change in growth: The excess of androgens leads to an acceleration of height and weight growth and bone maturation most often the height of children is greater than the normal average for their age bone maturation is accelerated, bone age is advanced compared to chronological age [11]. The excess weight is also noted, it is due to the muscular hypertrophy which accompanies virilism. The acceleration of bone maturation causes premature growth arrest by early closure of the epiphyses of the long bones.
Hypertension
This hypertension is explained by some authors by compression of the renal vessels by a large tumor [7]. Some observations show that HTA is present even in the case of a small tumor, this can be explained by the tumor secreting mineralocorticoids with a hypertensive effect [12].
In infants
Obesity: this is the first sign that sets in, it is most often a generalized obesity with a moon face, a bison hump, and rarely, it is similar to Cushingoid obesity of the adult. Arterial hypertension: for SABBAGA the hypertension is almost constant 90% of cases while C TEINTURIER only found it in 30% of cases [13]. Other signs are muscle deficit, diabetes and osteoporosis (less common than in adults), hirsutism. Cutaneous (acne, stretch marks) and neuropsychological signs are rare [14].
Feminizing tumors: 5.6. Feminizing tumors are
rare [12,15,16]. Conn Syndrome is very rare, few cases have been
reported in the literature, hypermineralocorticism is the cause
[17]. Regarding non-secreting tumors, they represent only 5% of
childhood ACCs. They have a poor prognosis because at the time
of diagnosis, the disease is often advanced. The clinical signs of
these tumors are linked [18], on the one hand, to the mechanical
phenomena generated by the invasion of neighboring structures;
on the other hand, to metastatic swarming. The diagnosis can be
made before abdominal pain, abdominal mass, weight loss. Other signs can be found digestive signs, general signs: anorexia, fever,
asthenia, hypertension of renal origin, inferior vena cava syndrome,
Budd Chiari syndrome.
The presence of metastases at diagnosis is uncommon in
children at 13%, unlike metastases in adults, which is 22 to 50%.
The standard non-hormonal biological workup may reveal nonspecific
abnormalities: an inconstant inflammatory syndrome.
Concerning hormonal biological signs, the biological diagnosis
of Cushing syndrome is made by the determination of serum
and urinary cortisol [19-21]. The assay of total testosterone is
recommended as a first-line treatment, the assay method chosen
must be specific and sensitive to assay low concentrations. DHEA
and SDHEA are the most specific. 90% of children with virilizing
ACC have a high plasma SDHEA level [7]. The dosage of urinary
17 ketosteroids is particularly interesting since it is high in
almost all patients with virilization syndrome [7]. Do not forget
to eliminate a pheochromocytoma by assaying plasma meta and
normetanephrines and urinary catecholamines. These hormonal
assays therefore make it possible to guide the diagnosis. It is
important to perform them before any treatment and in particular
before any surgical procedure, because their iterative performance
after treatment can make it possible to follow the evolution of the
disease and to detect early tumor recurrence in the event of reascension
after Initial normalization.
Radiologically; ultrasound cannot reliably detect even the
smallest adrenal lesions [21,22]. Thoraco-abdominopelvic
Computed Tomography (CT) is the gold standard [23,24]. ACC
appears on CT as a heterogeneous mass, with irregular margins
and a spontaneous density> 10 HU, the size of which exceeds 5cm.
It can present numerous calcifications, very often with necrotic or
hemorrhagic areas. This tumor enhances heterogeneously after
injection of the contrast product (42%). It presents a strong venous
tropism with possible extension to the renal vein or the inferior
vena cava. This examination also makes it possible to assess the
presence of a locoregional invasion (adipose tissue, mediastinalabdominal
and pelvic lymph nodes, vena cava, renal vein, adjacent
organs) or distant metastases (liver, lung, bone).
MRI can also complement abdominal CT data to refine the
assessment of locoregional, metastatic, vascular or lymph node
extension. It makes it possible to better define the relationships
with neighboring organs, in particular on the right with the liver
and above all it shows with precision the renal veins and the IVC up
to the right atrium and detects a tumor extension, with an almost
anatomical image, allowing true pre-surgical mapping [25]. On
MRI, the lesion appears hypointense in T1, hyperintense in T2;
sometimes there are very limited areas of intracytoplasmic fat. On
the out-of-phase sequences, an increase in the signal is obtained.
The extension workup is done with brain CT and bone scintigraphy.
Pathological analysis remains the cornerstone of the diagnosis
of adrenal tumors and in particular ACC. However, it remains a real
challenge, including for the most experienced pathologists for two
main reasons. The first difficulty is to establish with certainty the origin of the adrenal cortex of the lesion. For this, immunostaining
of Steroidogenic Factor 1 (SF-1) appears to be the most sensitive
and specific marker. The second difficulty is to discriminate the
benignity and the malignancy of the lesion [23]. The potential
for malignancy of an adrenal cortex tumor remains difficult to
establish, particularly in children. A different histopronostic score
than the adult Weiss score has been proposed by the Armed Forces
Institute Of Pathology (AFIP) for childhood tumors without being
completely reliable [26]. The classification of childhood ACC was
proposed by Sandrine and his team and modified from data from
the international registry and distinguishes 4 stages [27].
Preoperative preparation is essential in patients with
hypercriticism, tumor secretion leads to suppression and atrophy
of the contralateral gland; once the tumor is resected, adrenal
insufficiency sets in [28]. Surgery is the key, since “complete”
removal of a localized form, intra-adrenal tumor (stage II) or slightly
advanced (certain stages III), is the best chance of a real cure. The
presence of intraoperative tumor rupture increases the risk of
locoregional recurrence according to Sandrini and Michalkiewicz.
Ruptured tumors are therefore now considered stage III and
adjuvant therapy is currently recommended in these patients. This
rupture can also be spontaneous and be responsible for an acute
abdomen, or follow a needle biopsy performed for diagnostic
purposes. This gesture is therefore to be avoided. There is little data
in the literature on the incidence and role of lymph node invasion
in children because there are no clear recommendations for taking
lymph node samples during surgery. Based on experience at ST.
JUDE CHILDREN’S RESEARCH HOSPITAL, the presence of lymph
node invasion is associated with a poor prognosis.
The recommendation is to take lymph node samples at the
time of resection of the primary tumor and to perform lymph node
dissection when the result of the biopsy is positive [29]. The role of
chemotherapy is not yet clearly established in the management of
childhood ACC. The response rate varies between 7 and 54%, with
great variability in the response criteria [30]. Progress made in the
field of genomic analysis techniques and better knowledge of the
molecular players involved in the pathogenesis of ACC in adults but
also in children has made it possible to consider the use of so-called
targeted therapies. Faced with the absence of truly discriminating
anatomopathological prognostic markers, many studies have
shown the importance of clinical prognostic factors, such as tumor
size and weight. However, the thresholds set vary from study to
study.
Complete tumor resection is the most important prognostic
factor. Long-term survival is approximately 75% in children who
have had the tumor completely removed. Patients who have a
microscopic or macroscopic residue after surgery have a poor
prognosis [31]. Another factor is the type of tumor secretion:
children whose ACC secrete excess glucocorticoids seem to have
a poor prognosis, unlike those with pure signs of virilization [31].
The age at diagnosis appears to be an important prognostic factor.
Children under 2 years of age have a long-term survival rate of 82%
[8]. In contrast, children over 2 years of age have a survival rate of
29% [32]. The five-year overall survival rate of children with ACC
ranges from 54 to 74% depending on the series.
Conclusion
Adrenal Cortex Carcinoma (ACC) is a malignant tumor of the adrenal cortex that most commonly occurs in adulthood between the ages of 40 and 50. These tumors are extremely rare in children, corresponding to only 0.2% of childhood cancers. The sex ratio is in favor of girls. There are predisposing genetic factors in almost 50% of cases. The main mode of discovery of a SCC is the presence of endocrine signs by hormonal hypersecretion found ifn 80% to 90% of cases. Symptoms of virilization are most frequently observed. The diagnosis is based on clinical and paraclinical investigations and confirmed by pathological examination. The standard treatment for SCC is surgical excision, which should be complete if possible. When this resection is impossible or incomplete, and in metastatic forms, recourse to adjuvant or alternative treatment may be necessary. The prognosis of these tumors, as well as the survival rate, is very variable depending on the tumor stage.
References
- Ng L, Libertino JM (2003) Adrenocortical carcinoma: Diagnosis, evaluation and treatment. J Urol 169(1): 5-11.
- Michalkiewcz E, Sandrini B, Figueiredo B, Miranda EC, Caran E, et al. (2004) Clinical and outcome characteristics of children with adrenocortical tumors: A report from the international pediatric adrenocortical tumor registry. J Clin Oncol 22(5): 838-845.
- Cagle PT, Hough AJ, Pysher TJ, Johnson EH, Kirkland RT, et al. (1986) Comparison of adrenal cortical tumors in children and adults. Cancer 57: 2235-2237.
- Ribeiro RC, Figueiredo B (2004) Childhood adrenocortical carcinoma. Eur J Cancer 40(8): 1117-1126.
- Liou LS, Kay R (2000) Adrenocortical carcinomain children review and recent innovations. Urol Clin North Am 27(3): 403-421.
- Espiard S, Bertherat J (2015) The genetics of adrenocortical tumors. Endocrinol Metab Clin North Am 44(2): 311-334.
- Leblond P, Delebarre M, Aubert S (2011) Management of adrenocortical carcinomas in children. Bull Cancer 98(5): 595-605.
- Sabbaga CC, Avilla SG, Schulz C, Garbers JC, Blucher D (1993) Adrenocortical carcinoma in children: Clinical aspects and prognosis 28(6): 841-843.
- Teinturier C, Brugières L, Lemerle J, Chaussain JL, Bougnéres PF (2000) Child adrenal cortex retrospective analysis of 54 cases.
- Kumar S, Tiwari P, Kr Das R, Kr Kundu A (2010) Virilizing adrenal carcinoma in a 3-year-old boy: A rarity. Indian J Med Paediatr Oncol 31(1): 30-32.
- Burrington JD, Stephens CA (1969) Virilizing tumors of the adrenal gland in childhood: Report of eight cases. J Pediatr Surg 4(3): 291-302.
- Bouyahia O, Gharsallah L, Ouederni M, Boukthir S, Mrad SM, et al. (2009) Feminizing Adrenocortical Adenoma in a 5 Year-old Girl. J Pediatr Endocrinol Metab 22(1):79-84.
- Elisabethe B Fudge, Daniel L von Allmen (2009) Cushing syndrome in a 6-month-old infant due to adrenocortical tumor. Int J Pediatr Endocrinol 2009: 168749.
- Stratakis CA (2012) Cushing Syndrome in Pediatrics. Endocrinol Metab Clin North Am 41(4): 793-803.
- Ghazi AA, Mofid D, Rahimi F, Marandi H, Nasri H, et al. (1994) Oestrogen and cortisol producing adrenal tumour. Arch Dis Child 71(4): 358-359.
- Arnao JR, Perry L, Dacie JE, Reznek R, Ross RJ (1995) Primary hyperaldosteronism due to an adrenal adenoma in a 14-year-old boy. Postgrad Med J 71(832): 104-106.
- Bremont C, Luton lP (1998) Cushing syndromes. The practitioner's review, Paris monograph tome 48: 738-743.
- Libe R, Assie G (2014) Adrenal cortex: New in 2014.
- Plouin PF, Simon CM (1998) Primary hyperaldosteronism. Rev Prat 48(7): 749-753.
- Artus PM, Miquel C, Meria P, Hernigon A, Duclos JM (2004) Secreting adrenocortical tumors, Adrenocortical secretory tumors. Annals of Urology 38(4): 148-172.
- Rakoto-Ratsimba HN, Razafimahandry HJC, Ravalisoa A, Ranaivozanany A (2003) A case of giant malignant adrenocortical carcinoma. Ann Urol (Paris) 37(1): 17-20.
- Jebbari A, Nassar I, Edderai M, Bouklata S, Hammani L, et al. Principles of analysis of an adrenal mass: Contribution of CT and MRI. Radiology Department, Ibn Sina University Hospital, Rabat, Morocco.
- Oddoze C, Castinetti F (2013) Adrenal cortical pathologies and exploration of glucocorticoids 12(314).
- Benchekroun, Ghadouane, Alami M, Kassmaoui EH, Benslimane L, et al. (2000) Malignant adrenocortical carcinoma: About 22 cases. Advances in Urology 10: 205-210.
- (2010) Imageries of the adrenals. College of teachers of endocrinology, Diabetes and Diseases. Metabolics (CEEDMM).
- Wieneke JA, Thompson LDR, Heffess CS (2003) Adrenal cortical neoplasms in the pediatric population: A clinicopathologic and immunophenotypic analysis of 83 patients. Am J Surg Pathol 27(7): 867-881.
- Ribeiro RC, Pinto EM, Zambetti GP, Rodriguez-Galindo C (2010) The international pediatric adrenocortical tumor registry initiative: contributions to clinical, biological, and treatment advances in pediatric adrenocortical tumors. Mol Cell Endocrinol 351(1): 37-43.
- Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, et al. (2009) American association of clinical endocrinologists and american association of endocrine surgeons medical guidelines for the management of adrenal incidentalomas: Executive summary of recommendations. Endocr Pract 15(5): 450-453.
- Stewart JN, Flageole H, Kavan P (2004) A Surgical approach to adrenocortical tumors in children: The mainstay of treatment. Journal of Pediatric Surgery 39(5): 759-763.
- Baudin E, Leboulleux S, Al Ghuzlan A, Chougnet C, Young J, et al. Therapeutic management of advanced adre- nocortical carcinoma: what do we know in 2011? Horm Cancer 2(6): 361-371
- Ribeiro RC, Pinto EM, Zambetti GP (2010) Familial predisposition to adrenocortical tumors: Clinical and biological features and management strategies. Best Pract Res Clin Endocrinol Metab 24(2010): 477-490.
- Ciftci AO, Senocak ME, Tanyel FC, Büyükpamukçu N (2001) Adrenocortical tumors in children. J Pediatr Surg 36(4): 549-554.
© 2021 D Hanine. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






