- Submissions

Full Text
Research in Pediatrics & Neonatology
Protein S Deficiency and Pulmonary Embolism in Children: A Case Report and Review of Literature
Othman Rizk A Mishref*1, Abdelrahman A Beridan2 and Amr Abdelmordy E Samaka2
1Pediatrics & Neonatology Department, Kuwait Hospital, Kuwait
2Cardiology Department, Wara Hospital, Kuwait
*Corresponding author:Othman Rizk A Mishref, Pediatrics & Neonatology Department, Kuwait Hospital, Kuwait
Submission: December 23, 2020 Published: March 04, 2021

ISSN: 2577-9200 Volume5 Issue3
Abstract
Protein S deficiency is a major risk factor for venous thromboembolism. Unprovoked life-threatening pulmonary embolism is uncommon in children; however, it can pose a diagnostic challenge, presenting in a non-specific and subtle way, masked by lack of typical presentation. Therefore, it is important for pediatrician to consider pulmonary embolism as a differential diagnosis for young patients presenting with severe unexplained parasternal chest pain with strong family history of thrombophilia and to have a low threshold for requesting appropriate investigations. Here we present a young patient with acute pulmonary embolism who was finally diagnosed as Type III Protein S deficiency.
Keywords: Protein S deficiency; Pulmonary Embolism; Venous Thromboembolism; Thrombosis
Case Presentation
A previously healthy 12-year-old boy presented overnight to the accident and emergency
unit with dull aching left parasternal chest pain. The patient denied any history of trauma, heavy
muscular exercise, fever, chocking, cough, respiratory infection, drug intake, gastroesophageal
reflux disease, cardiac problems, or skin rash. His vital signs were normal, oxygen saturation
was 96% on room air, unremarkable systemic examination and chest x-ray was normal. This
patient is obese, his height is 155cm (>75th percentile), weight is 70kg (>95th percentile) and
BMI is 28.2. He was given nonsteroidal analgesic and sent home. Unfortunately, one hour
later, the patient came back with severe agonizing left parasternal chest pain associated
with difficult breathing in the form of tachypnea (respiratory rate, 32/m) and intercostal
retraction, tachycardia (heart rate, 120b/m), Blood pressure 100/60mmHg. Spo2 was 88%
in air, high-flow Oxygen 10L/m was applied by high flow mask, Spo2 improved to 98%, and
an arterial blood gas showed an evidence of type 1 respiratory failure (pH 7.43, pO2 56mmHg,
pCO2 30mmHg, Hco3 18.2).
ECG showed sinus tachycardia, evidence of right ventricular strain and suspected
pulmonary embolism in the form of SI, QIII, TIII (deep S wave in lead I, Q wave and inverted T wave
in lead III). The patient was given morphine 5mg/kg intramuscular. Urgent Echocardiography
was done and revealed mild tricuspid regurgitation. An urgent CT pulmonary angiogram was
requested and showed an evidence of sub-massive pulmonary embolism. The legs were free
of edema and there was no calf tenderness or erythema. Otherwise, systemic examination was
unremarkable. He had no previous thrombotic events. No past medical history of Neurological
disorder, stroke, slurring of speech, headache, blurring of vision, or loss of consciousness
or seizures. No history of gout, hypertension, diabetes mellitus, dyslipidemia, smoking or
chronic kidney disease. Tracing the family history, his uncle died suddenly at the age of 20
years old without any obvious cause and there is a history of hypercoagulability and venous
thromboembolic disease in the other branch of the family of the father, but he does not know
the exact cause, this information was missed initially by the father because of his anxiety.
Initial blood work on presentation showed a WBCs 11.3 x 103/
uL hemoglobin of 15g/dL (normal range: 13.5-17.5), HCT 44.6 %,
platelets 268 x 103/uL (normal range: 140-440), reticulocyte count
1.8 % (normal range: 0.5-2), Coagulation profile (APTT: 33.2 sec,
PT: 15.3 sec, Pc 84.9%, INR: 1.17, fibrinogen was 5.25 g/L, lactate
dehydrogenase 213U/L (normal range: 140-271), Peripheral
blood film was negative for schistocytes, and haptoglobin 132mg/
dL (normal range: 34-200mg/dL). COVID-19: Negative, CK-MB:
1.0 (N. 0.00- 4.3ng/mL), Troponin: 0.85 (N. 0.00- 0.40ng/mL)
Electrolytes, glucose, calcium, phosphorus, blood urea nitrogen
(BUN), creatinine, Liver Function Tests and lipid profile all were
normal. Urine analysis and microscopy were normal and there
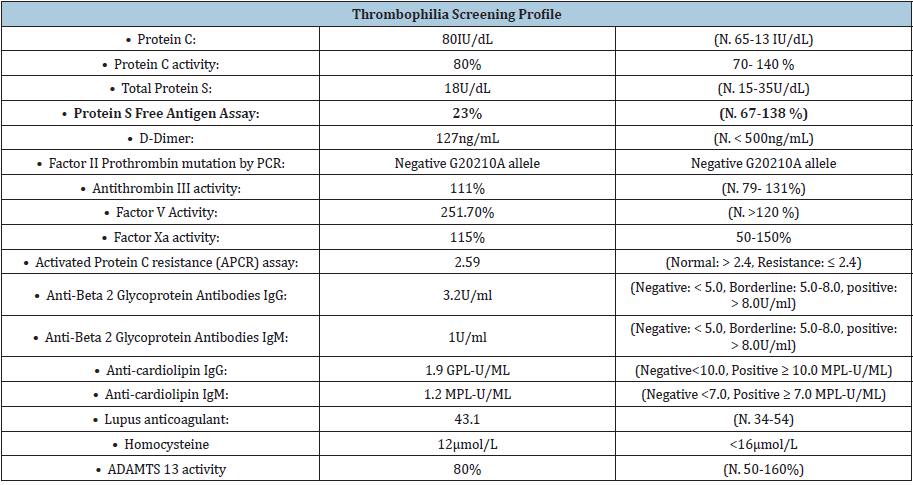
were no red blood cells. A thrombophilia screening profile of this
patient identified protein S deficiency (free protein S activity: 23%,
and normal total protein S level). The activity of protein C and
antithrombin III was within the normal limits (80% and 111%,
respectively). Other results, including levels of homocysteine,
factor II Prothrombin mutation G20210, factor V Leiden G1691A,
anti-cardiolipin antibody, and anti-β2-glycoprotein IgM and IgG, all
were negative. Therefore, a diagnosis of type III protein S deficiency
(low free protein S activity and normal total protein S level) was
established.
CT angiography showed picture suggestive of sub-massive
acute pulmonary embolism including segmental and sub-segmental
branches of both pulmonary arteries bilaterally (right more than
left). There is patchy consolidation of the basal segment of the left
lung with mild left pleural effusion. Furthermore, Leg veins color
Doppler ultrasound was done and demonstrated no evidence of
Deep Venous Thrombosis; moreover, in addition to, non-contrast
Brain CT showed no acute abnormalities. The patient’s father had
been thrombosis-free, but he was also found to have PS deficiency
with low PS activity (25%). No additional abnormalities were found
in any of the subjects after testing for anticardiolipin antibodies,
protein C level and activity, APCR-assay, factor V Leiden, and
prothrombin G20210A.
Coagulation profile was repeated after starting therapy (APTT:
35 sec, PT: 14.5 sec, Pc: 96.3%, INR: 1.2
Repeat WBCs 7.9 x 103/uL hemoglobin of 13.7g/dL (normal
range: 13.5-17.5), HCT 42 %, platelets 314 x 103/uL (normal
range: 140-440), lactate dehydrogenase 213U/L (normal range:
140-271), Peripheral blood film was negative for schistocytes, and
haptoglobin 152mg/dL (normal range: 34-200).
Differential Diagnosis
Due to the degree of hypoxia, family history of thrombophilia and right heart strain, massive pulmonary embolism remained the most likely diagnosis that was confirmed with High resolution CT pulmonary angiography chest Table 1. Other differentials were also considered, such as community-acquired pneumonia with pleurisy due to hypoxia and chest pain, but this did not explain the significant chest pain and the patient did not have fever, productive cough or patchy shadowing on chest x-ray. A patient presenting with shortness of breath and low oxygen saturations raises the possibility of a pneumothorax, but clinical examination and chest x-ray did not correlate with this diagnosis. Importantly, cardiac causes such as an inherited cardiomyopathy or connective tissue disease causing acute valvular pathology or dissection should remain a high differential diagnosis but ECG, Echocardiography, and CT pulmonary angiography excluded cardiac causes. Thrombophilia profile confirmed diagnosis of acute pulmonary embolism with Type III Protein S deficiency.
Table 1:
Treatment and Outcome
His symptoms and signs subsided after treatment with Enoxaparin 1mg/kg/dose, every 12 hours and then maintenance 1mg/kg once daily. He has also been offered genetic counselling advice in the future, should he wish to start a family. Hematology consultation was done and planned for follow up and genetic counselling for the family.
Background
Pulmonary embolism is defined as a blockage of a pulmonary
artery or one of its vessels by thrombus, fat, amniotic fluid or air.
A massive pulmonary embolism is characterized by hypotension,
shock, right ventricular dysfunction and/or myocardial injury [1].
Venous thromboembolism (VTE) in a young, healthy patient is
uncommon in the absence of a provoking factor. VTE occurs when
≥1 component of Virchow’s triad is activated: stasis of blood flow,
injury to the endothelial lining, and hypercoagulability of blood
components. This is the most useful pathophysiological construct
for thinking about thromboembolism in children [2]. Protein S
(PS) deficiency contributes to 2% of all venous thromboembolisms
presenting to accident and emergency and its deficiency is a major
risk factor for venous thrombosis [3]. Studies of families with
thrombophilia revealed that individuals with PS deficiency have a
five to ten-folds higher risk for VTE than healthy relatives [4].
PS, a vitamin K–dependent glycoprotein, circulates in plasma in
two forms: a complex with C4b-binding protein (bound form 60 %
of total PS) and, in part as the functionally active form “free” protein
S (40 %) [5]. PS, mainly synthesized in the liver and endothelial
cells, is a cofactor of activated protein C (PC). The free form serves
as cofactor with APC to inactivates factor FVa and FVIIIa, thereby
restricting thrombin generation. Furthermore, the anticoagulant
activity of PS might act independently from PC via an additional
non-enzymatic cofactor function for tissue factor pathway inhibitor
thereby promoting inhibition of FXa. Additionally, PS plays a direct
role in inhibition of thrombin generation via inhibition of FXa and
FVa in the prothrombinase complex [6].
PS deficiency is detected using tests for PS antigen (total antigen
or free PS antigen). Decreased levels of PS are found in inherited
deficiency of PS as well as in some acquired conditions, such as liver
diseases, vitamin K deficiency, therapy with vitamin K antagonists,
pregnancy, HIV infection, varicella, sickle cell disease, malignancy,
and nephrotic syndrome [7].
Protein S deficiency (PSD) usually presents as heterozygous
deficiency and segregates as an autosomal dominant trait.
Heterozygous carriers may manifest at a later age; meanwhile,
homozygous PSD can lead to an early thrombotic onset in the
neonatal or infant period. According to plasma levels of total PS,
free PS antigen and PS activity, PS deficiency is classified into 3
types; Type I is a quantitative deficiency in the total PS and free
PS. Type II (qualitative PS deficiency) is characterized by normal PS
levels but reduced PS activity due to a dysfunctional PS variant in
plasma. Type III PS deficiency is characterized by low levels of free
PS, though the total plasma concentration of PS is normal [8].
The inherited PS deficiency is an autosomal dominant condition.
Molecular studies identified two genes encoding PS, linked closely
on chromosome 3p11.1-3q11.2: the active gene, PROS-b (PROS1)
and the other, the pseudogene PROS-a, a nonfunctional gene, similar
with the active gene, but without the exon 1. Almost 200 mutations
of PROS1 gene have been described, such as: the PS Heerlen
mutation (due to the substitution of Ser460 by Pro, consecutive to
T/C transition in exon 13) resulting in type III deficiency, the PS
Tokushima due to K196E substitution, located in the epidermal
growth factor-2 domain and causing type II deficiency [3]. LMWH
has become the anticoagulant of choice in many pediatric patients
for a variety of reasons. The most reported LMWH used in pediatric
patients is enoxaparin. LMWH may require weekly monitoring
of anti-Xa levels in children in the inpatient setting requiring
therapeutic Xa levels. In asymptomatic individuals with PS
deficiency, the prophylaxis of VTE should be done in the presence
of a major acquired risk factor for thrombosis [9].
Discussion
Unprovoked life-threatening pulmonary embolism is
uncommon in children. They can pose a diagnostic challenge,
presenting in a non-specific and subtle way, masked by lack of
typical presentation. Interestingly, in the case described above,
the patient presented with pulmonary embolism without any
other acquired risk factors, indicating a potential abnormality in
anticoagulation mechanisms. In our opinion, children presented
with pulmonary embolism in the absence of precipitating factors
need to be tested to identify whether there is a deficiency of PC, PS,
or antithrombin III (AT III). In this case, the patient was found to be
PS deficient.
Current evidence indicates that screening for inherited
thrombophilia is appropriate in cases of VTE without obvious cause
for VTE in patients with a positive family history of thrombosis;
recurrent VTE; thrombosis at an unusual location; and developing
VTE during pregnancy, use of oral contraceptives, or hormone
replacement therapy [10]. Therefore, it is important for pediatrician to consider pulmonary embolism as a differential diagnosis for
young patients and have a low threshold for requesting appropriate
investigations. Virchow’s triad describes three primary causes of
venous and arterial thrombosis: abnormalities in the circulating
blood, stasis and injury to the vessel wall [11]. Hereditary
thrombophilias are associated with blood abnormalities and are
characterized by venous thromboembolic events at a young age.
These are often unprovoked and recurrent, occurring at unusual
locations and associated with a family history. Protein S deficiency
predisposes to higher risk of venous thromboembolism than the
general population with normal protein S level [12].
Obtaining a thorough family history on admission is very crucial
to aid diagnosis. The patient presented with significant hypoxia
and right ventricular strain suspicious of pulmonary embolism. An
urgent CT pulmonary angiogram, the main investigative modality on
admission, was requested and revealed the life-threatening extent
of pulmonary embolism, allowing immediate treatment. As this was
the case with our patient, having a family history of thrombophilia
prompted to investigate for hereditary thrombophilia including
protein S deficiency. In patients with a coexisting clinical deep vein
thrombosis, leg color Doppler ultrasound as the initial imaging test
can be sufficient to confirm venous thromboembolism [13]. But
this was not there in our case. To prove inherited deficiency, testing
of family members is recommended. Both patient and family
members should receive genetic counseling prior to genetic testing,
and such testing should only be performed after obtaining consent.
Interestingly, the patient’s father had also PS deficiency, but was
thrombosis-free, suggesting that a triggering event might have
been involved in the patient’s thrombus formation or the existence
of a protective mechanism in the patient’s father.
Summary
In summary, our report supports the view that PS deficiency should be taken into consideration for children with pulmonary embolism presenting with unexplained chest pain without obvious predisposing factors. Family history is extremely important in such cases and genetic studies could be valuable in the identification of PROS1 mutations for the PS deficient patients. Following the confirmation of massive pulmonary embolism, the patient received enoxaparin and genetic testing for the family is requested.
Learning Points
A. Protein S deficiency is a rare but important cause for venous
thromboembolism in children.
B. A thorough family history should be incorporated into the
medical clerking of all patients admitted to the accident and
emergency.
C. Knowledge of the presence of hereditary illnesses, such
as thrombophilia, provides a very significant guidance in
creating differential diagnoses, prioritizing investigations and
providing the best care for the patient.
D. Pulmonary emboli can present with non-specific symptoms.
Meticulous clinical assessment of the patient as well as a low
threshold for investigation on admission can be lifesaving.
E. children presenting with unprovoked pulmonary emboli,
clinicians should consider inherited prothrombotic factors as
a potential cause. A thrombophilia screen not only allows for
better management of the patient, but also enables diagnosis
of family members.
F. DNA-based testing is a useful diagnostic approach to diagnose
protein S deficiency when more than one family member is
affected with thrombosis.
References
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, et al. (2008) Guidelines on the diagnosis and management of pulmonary embolism: The task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology(ESC). Eur Heart J 29(18): 2276-315.
- Witmer CM, Takemoto CM (2017) Pediatric hospital acquired venous thromboembolism. Front Pediatr 5: 198.
- Ten Kate MK, Der Meer JV (2008) Protein S deficiency: A clinical perspective. Haemophilia 14(6): 1222-1228.
- Yin T, Miyata T (2009) Venous thromboembolic risk and protein S deficiency: Ethnic difference and remaining issues J Geriatr Cardiol 6(1): 11-19.
- Dahlbäck B (2007) The tale of protein S and C4b-binding protein, a story of affection. Thromb Haemost 98(1): 90-96.
- Castoldi E, Simioni P, Tormene D, Rosing J, Hackeng TM (2010) Hereditary and acquired protein S deficiencies are associated with low TFPI levels in plasma. J Thromb Haemost 8(2): 294-300.
- Matthes BK (1992) Acquired protein S deficiency. Clin Investig 70(6): 529-534.
- Martinelli I, Bucciarelli P, Artoni A, Fossali EF, Passamonti SM (2013) Anticoagulant treatment with rivaroxaban in severe protein S deficiency. Paediatrics 132(5): e1435-1439.
- Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, et al. (2012) Antithrombotic therapy in neonates and children: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 141(2 suppl): e737S-e801S.
- Wypasek E, Undas A (2013) Protein C and protein S deficiency: Practical diagnostic issues. Adv Clin Exp Med 22(4): 459-67.
- Khan S, Dickerman JD (2006) Hereditary thrombophilia. Thromb J 4: 15.
- Dykes AC, Walker ID, McMahon AD, Islam SI, Tait RC (2001) A study of protein S antigen levels in 3788 healthy volunteers: Influence of age, sex and hormone use, and estimate for prevalence of deficiency state. Br J Haematol 113(3): 636-634.
- British thoracic society standards of care committee pulmonary embolism guideline development group (2003) British thoracic society guidelines for the management of suspected acute pulmonary embolism. Thorax 58(6): 470-84.
© 2021 Othman Rizk A Mishref. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






