- Submissions

Full Text
Research in Pediatrics & Neonatology
Perioperative Echocardiographic Hemodynamic Parameters and Postoperative Outcome in Pediatric Surgical Patients: A Descriptive Observational Prospective Pilot Study Protocol
Kumba C1,2,3,4* and Tréluyer JM 2,3
1Department of Pediatric Anesthesia and Critical Care, Necker Enfants Malades Hospital,University of Paris
2Department of Clinical Research and Pharmacology, Necker Enfants Malades and Cochin University Hospitals, Assistance Publique Hôpitaux de Paris, Paris Descartes University,University of Paris
3EA-7323 Pharmacologie et Evaluation des Thérapeutiques Chez L ‘Enfant et La Femme Enceinte, Université Paris Descartes, Université de Paris, Paris, France
4Ecole Doctorale 563 Médicaments-Toxicologie-Chimie-Imageries (MTCI), Université Paris- Descartes, Université de Paris, Paris, France
*Corresponding author:Claudine Kumba, Department of Pediatric Anesthesia and Critical Care, Necker Enfants Malades University Hospital
Submission: December 16, 2019; Published: December 19, 2019

ISSN: 2577-9200 Volume4 Issue1
Abstract
Background: A randomized controlled trial (RCT) protocol in pediatric patients scheduled for surgery will be elaborated. In this RCT protocol trans-thoracic echocardiography will be realized perioperatively to guide fluid and hemodynamic therapy in these patients. This RCT will determine the impact of goal directed therapy with echocardiography on postoperative outcome in terms of morbidity, Length of Intensive Care Unit Stays (LOSICU), Length of Mechanical Ventilation (LMV), Length of Hospital Stays (LOS), fluid therapy and vasopressor-inotropic therapy. There are no trials in pediatric surgical patients which have identified echocardiographic hemodynamic parameters predictive of postoperative outcome in terms of morbidity, LOSICU, LMV and LOS. The objective of this pilot observational prospective trial protocol is to describe the study which will determine echocardiographic hemodynamic parameters predictive of postoperative outcomes. These hemodynamic parameters will be integrated in the RCT which has the objective to determine the impact of goal directed fluid and hemodynamic therapy guided by trans-thoracic echocardiography on postoperative adverse outcome.
Methods: Patients aged less than 18 years admitted for surgery will be included. Trans-thoracic echocardiography will be realized to measure different hemodynamic parameters perioperatively in included patients. Primary outcome will be postoperative morbidity, secondary outcomes will be LOSICU, LMV and LOS; tertiary outcomes will be fluid therapy, vasopressor and inotropic therapy. Primary outcome measure will be the presence of postoperative organ dysfunction. Secondary outcome measures will be the number of postoperative days spent in the Intensive Care Unit (ICU), number of postoperative days spent on invasive or non-invasive mechanical ventilation and the number of postoperative days spent in the conventional hospitalization ward. Tertiary outcome measures will be the quantity of fluid administered and the Vasopressor-Inotropic Score (VIS). The study will be monocentric. XLSAT 2018.3 or plus will be the software for statistical analysis. Results are expected in the first semester of 2022.
Conclusion: This pilot study will identify echocardiographic hemodynamic parameters predictive of postoperative adverse outcome which will be integrated in the second RCT where goal directed fluid and hemodynamic therapy will be guided with echocardiography.
Keywords: Pediatric surgery; Children; Echocardiography; Hemodynamics; Fluid therapy; Postoperative outcome
Introduction
Perioperative goal directed fluid and hemodynamic therapy (PGDFHT) has been studied in adults where it has demonstrated its efficacy in terms of reduced postoperative complications and length of hospital stay (LOS) [1-7]. The objective of PGDFHT is to monitor fluid responsiveness and hemodynamic status with the aim to improve oxygen delivery to different systemic organs and to improve tissular perfusion [8]. Tissular hypoperfusion can have side effects in terms of organ failure. Unoptimal fluid and hemodynamic status (insufficient or plethoric) can alter tissular perfusion. Therefore, monitoring fluid responsiveness and hemodynamic status using tools to assess adequate cardiac output to maintain sufficient tissular oxygen delivery is mandatory. There are no studies in children demonstrating the impact of PGDFHT with echocardiography on postoperative outcome. However, there are studies in pediatric cardiac surgery mostly which identified perioperative biomarkers of postoperative adverse outcome [9].
These biomarkers were lactate levels, central venous oxygen saturation SCVO2, regional cerebral, renal, splanchnic oxygen saturation and veno-arterial carbon dioxide gradient. Unoptimal values of these biomarkers predicted adverse postoperative outcome in terms of mortality, morbidity and length of hospital stay (LOS) [9]. Concerning the tool to assess cardiac output, fluid responsiveness and hemodynamic status, transthoracic echocardiography is a noninvasive mean which can bring solutions and some parameter like the variation of peak velocity at the aortic annulus has been validated to predict fluid responsiveness in children [10]. There are no studies which have clarified echocardiographic hemodynamic parameters predictive of postoperative outcome in children scheduled for surgery in general. Nevertheless, there is one retrospective study in pediatric and adult cardiac surgery which showed that intraoperative transesophageal echocardiography after surgical repair in congenital heart disease reduced LOS [11]. We have elaborated an RCT trial where fluid and hemodynamic therapy will be guided with trans-thoracic echocardiography. In this RCT echocardiography hemodynamic parameters will be integrated in a protocol to guide fluid, inotropic and or vasopressive therapy. To validate these echocardiographic hemodynamic parameters, we will conduct a pilot observational prospective study to identify those which are predictive of postoperative adverse outcome. We describe here this pilot trial. We have elaborated 2 similar protocols in pediatric patients with congenital heart disease [12,13]. We would like to generalize the protocol to other pediatric surgical patients. The primary objective of this study protocol is to describe the pilot trial which will be undertaken to identify echocardiographic hemodynamic parameters predictive of postoperative outcome in terms of morbidity.
The secondary objective is to clarify echocardiographic hemodynamic parameters predictive of postoperative LOSICU, LMV and LOS. The tertiary objectives are to determine echocardiographic hemodynamic parameters predictive of fluid therapy, vasopressor and inotropic therapy. The primary outcome measures will be postoperative organ dysfunction until discharge from hospital. The secondary outcome measures will be the number of postoperative days spent in the Intensive Care Unit (ICU), the number of postoperative days spent on invasive or noninvasive mechanical ventilation and the number of postoperative days spent in the conventional hospitalization ward. The tertiary outcome measures will be the quantity of postoperative fluid administered in terms of crystalloids, colloids, blood product and postoperative vasopressor inotropic score. Once the echocardiographic hemodynamic parameters predictive of postoperative outcome have been identified in this pilot study, they will be integrated in a randomized controlled trial which will determine the impact of intraoperative goal directed therapy with echocardiography in general pediatric surgery on postoperative outcome.
Methods and Materials
This trial has been declared at the French National Agency of Drugs and Medications Security, ANSM (National Agency for The Safety of Medicines and Health Products) and registered under the number RCB: 2019-A03256-51. After approval from the Ethics Committee, and after parents and or patient’s information, patients will be included prospectively in one cohort. The patients included will be managed according to the usual local practices. Echocardiography Figure 1 will be realized in each patient perioperatively after induction of anesthesia. The echocardiographic hemodynamic parameters measured are precised below. The patients included will be children aged less than 18 years admitted for surgery or other intervention under anesthesia. General variables registered will be age, gender, type of surgery, elective or urgent surgery, American Society of Anesthesiologists status (ASA), weight, height, prematurity, blood pressure, heart rate, pulse oximetry and hemoglobin levels. Preoperatively basal values of blood pressure, heart rate, pulse oximetry, body temperature will be registered prior to anesthesia and surgery and intraoperatively during surgery hourly. Other intraoperative parameters registered are blood product transfusion (Packed Red Blood Cells (PRBC), Fresh Frozen Plasma (FFP), Concentrated Platelet Units (CUP), fibrinogen, cryoprecipitate, Concentrated Complex of Prothrombin (CPP) or other blood product derivatives, crystalloids and colloids or other fluids administered, blood loss, urinary output, quantity of inotrops, diuretics, anesthetic drugs administered and mechanical ventilation parameters, central venous pressure if monitored. Normal blood pressure and heart rate values are those defined according to the patient age [14]. In addition to echocardiographic hemodynamic parameters, postoperative variables registered once daily until discharge from hospital will be blood pressure, heart rate, core temperature, pulse oximetry, CVP if monitored, blood product transfusion (PRBC, FFP, CUP), fibrinogen, cryoprecipitate, concentrated complex of prothrombin, other blood product derivatives, crystalloids, colloids or other fluids administered, blood loss, urinary output, quantity of inotropes, diuretics, anesthetic drugs administered, mechanical ventilation parameters, hemoglobin levels. Trans-thoracic echocardiographic parameters measured perioperatively are described here after. Since echocardiography is operator dependent, all the echocardiographic hemodynamic parameters will be measured by one experienced medical doctor in pediatric echocardiography and validated by a second experienced medical doctor. Cardiac output measures will be realized with Velocity Time Integral (VTI) at the aortic valve in the apical five chamber view. Normal values of aortic VTI have been defined in children [15].
Fluid responsiveness will be assessed with aortic peak velocity at the apical five chambers view with peak velocity variation (ΔVpeak) of ≥10% defining responders to fluid therapy. ΔVpeak is defined as (Vmax-Vmin ⁄[(Vmax+Vmin)2]) X 100 (10). Right ventricular (RV) and left ventricular (LV) systolic function will be assessed in the apical four chamber view with lateral S (Slat) wave velocity in tissue Doppler, with mitral and tricuspid annular plane systolic excursion (MAPSE, TAPSE) in time motion mode (TM) and with ejection fraction EF with Simpson’s method. Normal MAPSE, TAPSE and Slat values have been defined in children [16-21]. Fractional shortening (FS) will be assessed in the parasternal longitudinal axis view, normal values are the same as in adults (28-42%). Right ventricular and left ventricular diastolic function will be assessed in the apical four chamber view at the tricuspid and mitral valves with pulsed Doppler to assess for E wave velocity, A wave velocity and E/A ratio. E/A ratios will be analyzed according to age [22- 29]. To assess for normal, relaxation alteration, pseudonormal and restrictive profiles. Right and left filling pressures will be assessed with tissue Doppler at the apical four chamber view at the tricuspid and mitral valves to assess lateral E’ wave velocity and E/E’lat ratio.
Figure 1:Echocardiographic hemodynamic parameters.
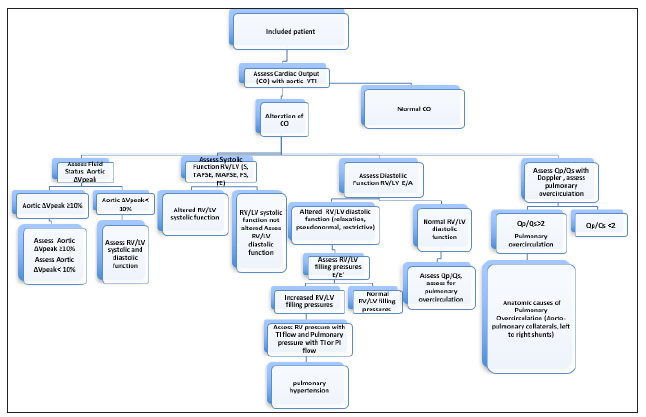
Normal E/E’ and E’lat values have been defined in children [22- 29]. To assess for pulmonary over circulation, Qp/Qs ratio (where Qp is pulmonary output and Qs is systemic cardiac output) will be calculated using the formula Qp/Qs= Pulmonary VTI x Area of the pulmonary annulus x HR /Aortic VTI x Area of the aortic annulus x HR= VTIp x IIx (D/2)2/VTIao x IIx (D/2)2, where D is the diameter of the annulus and HR the heart rate [30]. Pulmonary VTI and pulmonary annulus diameter will be assessed at the parasternal transverse axis view. Aortic VTI will be assessed at the apical 5 chamber view and the aortic annulus diameter at the parasternal longitudinal axis view. The inferior veina cava diameter (IVC) and the variation of the latter (ΔIVC) will be assessed at the subcostal view and will be defined as Δ IVC=[(Dmax-Dmin/(Dmax+Dmin/2)]x100. Where Dmax is the maximum and Dmin is the minimum diameter of the IVC. Supra-hepatic Doppler waves velocity V, A, S, D and S/D ratios will be assessed in the subcostal view. Pulmonary Doppler waves velocity S, D, E, Ap and S/D ratios will be assessed in the apical four chamber view. Postoperative organ dysfunction until discharge from hospital will be registered to assess for primary outcome. The number of days spent in ICU, under invasive or noninvasive mechanical ventilation and days in the conventional hospitalization ward postoperatively will be registered to assess for secondary outcomes. The quantity of postoperative fluid (crystalloids, colloids, blood products) administered and postoperative vasopressor inotropic score will be registered to assess for tertiary outcomes. Statistical analysis will be realized with XLSTAT 2018.3 or plus software. Normally distributed and non-normally distributed variables will be compared using Student t or Mann-Whitney tests and Wilcoxon or Kruksal-Wallis tests respectively.
Normally distributed variables will be expressed in terms of means with standard deviation. Non normally distributed variables will be expressed in terms of medians with interquartile ranges. Categorical variables will be compared with the exact Fisher’s test or Chi squared test accordingly. Categorical variables will be expressed as percentages with 95% confidence intervals. To assess for independent predictors of adverse postoperative outcome, multivariate analysis will be realized. A P-value≤0.05 will be considered significative. Missing data will not be included. The study is expected to begin first semester of 2021 and will terminate end 2021. The number of patients included will be 1000 patients to have a normally distributed population. The study will be monocentric.
Result
Results are expected in the first semester of 2022.
Conclusion
This study protocol was designed to describe the pilot observational prospective trial which will identify echocardiography parameters predictive of postoperative outcome in terms of morbidity, LOSICU, LMV, LOS, fluid therapy and vasopressor inotropic score in children scheduled for surgery or other interventions. These echocardiographic predictors of the abovementioned outcomes will be integrated in randomized controlled mono-multicentric trials which will determine the impact of intraoperative goal directed fluid and hemodynamic therapy with echocardiography on postoperative outcome in children scheduled for surgery or other interventions under general anesthesia.
Disclosure
This study is part of the Thesis entitled ‘Do goal directed therapies improve postoperative outcome in children? (Perioperative Goal Directed Fluid and Hemodynamic Therapy; Transfusion goal directed therapy using viscoelastic methods and enhanced recovery after surgery and Postoperative outcome)’ [31-33]. This Thesis is registered online http://www.theses.fr/ s232762.
References
- Sun Y, Chai F, Pan C, Romeiser JL, Gan TJ (2017) Effect of perioperative goal-directed hemodynamic therapy on postoperative recovery following major abdominal surgery-a systematic review and meta-analysis of randomized controlled trials. Crit Care 21(1): 141.
- Giglio M, Marucci M, Testini M, Brienza N (2009) Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth 103(5): 637-646.
- Aya H, Cecconi M, Hamilton M, Rhodes A (2013) Goal-directed therapy in cardiac surgery: a systematic review and meta-analysis. Br J Anaesthesia 110(4): 510-517.
- Dalfino L, Giglio M, Puntillo F, Marucci M, Brienza N (2011) Haemodynamic goal-directed therapy and postoperative infections: earlier is better. A systematic review and meta-analysis. Critical Care 15(3): R154.
- Giglio M, Dalfino L, Puntillo F, Rubino G, Marucci M, et al. (2012) Haemodynamic goal-directed therapy in cardiac and vascular surgery. A systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 15: 878-887.
- Giglio M, Manca F, Dalfino L, Brienza N (2016) Perioperative hemodynamic goal-directed therapy and mortality: a systematic review and meta-analysis with meta-regression. Minerva Anestesiol 82(11): 1199-1213.
- Chong M, Wang Y, Berbenetz N, McConachie I (2018) Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes? A systematic review and meta-analysis. Eur J Anaesthesiol 35(7): 469-483.
- Lemson J, Nusmeier A, Van der Hoeven J (2011) Advanced hemodynamic monitoring in critically ill children. Pediatrics 128(3): 560-571.
- Kumba C, Willems A, Querciagrossa S, Harte C, Blanc T, et al. (2019) A systematic review and meta- analysis of intraoperative goal directed fluid and haemodynamic therapy in children and postoperative outcome. J Emerg Med Critical Care 5(1): 9.
- Pereira de Souza Neto E, Grousson S, Duflo F, Ducreux C, Joly H, et al. (2011) Predicting fluid responsiveness in mechanically ventilated children under general anaesthesia using dynamic parameters and transthoracic echocardiography. Br J Anaesth 106(6): 856-864.
- Madriago E, Punn R, Geeter N, Silverman NH (2016) Routine intra-operative trans-oesophageal echocardiography yields better outcomes in surgical repair of CHD. Cardiol Young 26(2): 263-268.
- Kumba C, Raisky O, Bonnet D, Treluyer JM (2019) Perioperative goal directed fluid and hemodynamic therapy with echocardiography in pediatric congenital heart disease: a study protocol. EC Paediatrics 8(12): 01-06.
- Kumba C, Raisky O, Bonnet D, Tréluyer JM (2019) Perioperative echocardiographic hemodynamic parameters and postoperative outcome in pediatric congenital heart disease: a descriptive observational prospective pilot study protocol. Int J Pediatr Neonat Care 5: 160.
- de Graaff JC, Pasma W, van Buuren S, Duijghuisen JJ, Nafiu OO, et al. (2016) Reference values for noninvasive blood pressure in children during anesthesia. a multicentered retrospective observational cohort study. Anesthesiology 125(5): 904-913.
- Solinski A, Klusmeier E, Horst JP, Körperich H, Hass AN, et al. (2018) Centile curves for velocity-time integral times heart as a function of ventricular length: the use of minute distance is advantageous to enhance clinical reliability in children. J Am Soc Echocardiogr 31(1): 105-112e.
- Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, et al. (2009) Right ventricular function infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (tapse) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr 22(6): 715-719.
- Terada T, Mori K, Inoue M, Yasunobu H (2016) Mitral annular plane systolic excursion/left ventricular length (MAPSE/L) as a simple index for assessing left ventricular longitudinal function in children. Echocardiography 33(11): 1703-1709.
- Koestenberger M, Nagel B, Ravekes W, Avian A, Cvirn G, et al. (2014) Reference values of the mitral annular peak systolic velocity (sm) in 690 healthy pediatric patients, calculation of z-score values, and comparison to the mitral annular plane systolic excursion (MAPSE). Echocardiography 31(9): 1122-1130.
- Koestenberger M, Nagel B, Ravekes W, Avian A, Heinzl B, et al. (2102) Left ventricular long-axis function: reference values of the mitral annular systolic excursion in 558 healthy children and calculation of z-score values. Am Heart J 164(1): 125-131.
- Eiden B, McMahon C, Cohen RR, Wu J, Finkelshteyn I, et al. (2004) Impact of cardiac growth on doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr 17(3): 212-221.
- Frommelt PC, Ballweg JA, Whitstone BN, Frommelt MA (2002) Usefulness of doppler tissue imaging analysis of tricuspid annular motion for determination of right ventricular function in normal infants and children. American Journal of Cardiology 89(5): 610-613.
- Lester SJ, Tajik J, Nishimura RA, Oh JK, Khandheria BK (2008) Unlocking the mysteries of diastolic function. deciphering the rosetta stone 10 years later. J Am Coll Cardiol 51(7): 679-689.
- Vitarelli A, Conde Y, Cimino E, D Angeli I, D Orazio S, et al. (2005) Quantitative assessment of systolic and diastolic ventricular function with tissue doppler imaging after fontan type of operation. Int J Cardiol 102(1): 61-69.
- Ichihashi K, Sato A, Shiraishi H, Momoi M (2011) Tissue doppler combined with pulsed-wave doppler echocardiography for evaluating ventricular diastolic function in normal children. Echocardiography 28(1): 93-96.
- Cantinotti M, Giordano R, Scalese M, Murzi B, Assanta N, et al. (2016) Normograms for mitral inflow doppler and tissue doppler velocities in caucasian children. J Cardiol 68(4): 288-299.
- Dallaire F, Slorach C, Hui W, Sarkola T, Friedberg MK, et al. (2015) Reference values for pulse wave doppler and tissue doppler imaging in pediatric echocardiography. Circ Cardiovasc Imaging 8(2): e002167.
- Harada K, Orino T, Yasuoka K, Tamura M, Takada G (2000) Tissue doppler imaging of left and right ventricles in normal children. Tohoku J Exp Med 191(1): 21-29.
- Lee A, Nestaas E, Brunvand L, Liestøl K, Brunvand L, et al. (2014) Tissue doppler imaging in very preterm infants during the first 24h of life: an observational study. Arch Dis Child Fetal Neonatal 99(1): F64-F69.
- Mi YP, Abdul-Khaliq H (2013) The pulsed doppler and tissue doppler-derived septal E/e’ ratio is significantly related to invasive measurement of ventricular end-diastolic pressure in biventricular rather than univentricular physiology in patients with congenital heart diasease. Clin Res Cardiol 102(8): 563-570.
- Klimczak C (2010) Clinical echocardiography (6th ), Elsevier Masson, Paris, France, pp. 32-34.
- Kumba C, Mélot C (2019) The era of goal directed therapies in paediatric anaesthesia and critical care. EC Emergency Medicine and Critical Care 3(5): 306-309.
- Kumba C (2019) Do goal directed therapies improve postoperative outcome in children? (Perioperative goal directed fluid and hemodynamic therapy; transfusion goal directed therapy using viscoelastic methods and enhanced recovery after surgery and postoperative outcome): A study research protocol. Acta Scientific Paediatrics 2(7): 17-19.
- Kumba C (2019) Future evolution of intraoperative goal directed fluid and hemodynamic therapy in Children. Adv Pediatr Res 6(2): 1-2.
© 2019 Harroche Annie. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






