- Submissions

Full Text
Research in Pediatrics & Neonatology
Correlation of Transcutaneous Bilirubin and Total Serum Bilirubin after Photo Therapy where a Definite Spot of Skin Remains Patched in Case of Indian Brownish Complexioned Babies
Mittal M1, Raktima Chakrabarti2*, Balde M2, Yadav BS2 and Wazir S2
1Department of Neonatology, Cocoon Hospital, India
2Department of Neonatology, Cloudnine Hospital, India
*Corresponding author: Dr Raktima Chakrabarti, Department of Neonatology, Cloudnine Hospital, India, Email: dr_raKtima@yahoo.com
Submission: December 18, 2017;Published: May 23, 2018

ISSN: 2576-9200 Volume2 Issue2
Abstract
Aim: To study the correlation between serum bilirubin (TSB) and transcutaneous bilirubin (TcB) after phototherapy on an area kept covered during the phototherapy treatment in Indian brownish complexioned newborns, with an aim to reduce post-therapy blood sampling.
Till now, no difference is found between Caucasian and brownish newborns in response to phototherapy. Previously TcB and TSB value correlation studies were done in Caucasian population but in case of Indian brownish complexion babies these correlations were not studied. So, our endeavor is to assess these correlations to decrease needle pricks in newborn.
Methods: 100 Late preterm (>35 weeks gestational age) and term neonates requiring phototherapy for the first time were enrolled in this study. Pre and post phototherapy (after 24 hours of therapy), TcB was assessed for all babies over mid sternum and on forehead. TSB was also determined pre and post phototherapy. The point on mid sternum where TcB was assessed was patched (area of 2.5cm diameter) with photo-opaque material before starting of phototherapy. The decision to initiate and stop phototherapy was as per the AAP recommendations.
Results: Post-therapy, significant correlation between TcB at both patched and unpatched area with TSB was found but significance level was much higher (less no of outliers) for the patched area. Analysis showed, post-therapy TcB level on patched area <10.1mg/dl is a good indicator to stop phototherapy without a TSB.
Conclusion: Transcutaneous bilirubinometry over a shielded area can give a fair idea of the declining trend in bilirubin levels, to avoid pricking the babies for TSB prior to stopping phototherapy.
Keywords: Neonatal jaundice; Phototherapy; Photo-opaque; Serum bilirubin; Transcutaneous bilirubinometry
Abbreviations: TSB: Total Serum Bilirubin; TcB: Transcutaneous Bilirubin; AAP: American Academy of Pediatrics; ROC: Receiver Operating characteristics
Introduction
Jaundice is observed during the first week of life in about 60% of term and 80% of preterm babies [1]. Yellow discoloration is first evident on the skin of face, nasolabial fold and tip of the nose. Nearly 10% of this term jaundiced neonates require phototherapy [2]. Once on phototherapy, they require repeated blood sampling, which is painful, stressful and poses increased risk of infections [3,4]. This method in addition is time consuming and laborious [5].
Transcutaneous bilirubinometry is an easy, safe and convenient method for evaluation of neonates with jaundice [6-10]. Transcutaneous bilirubin (TcB) may reduce the overall blood draw rate by 20% [10]. In addition, it bears a linear correlation with total serum bilirubin (TSB) except under some special circumstances [13-15]. Hence the use of transcutaneous bilirubinometers has been recommended in clinical practice guidelines in the management of hyerbilirubinemia [16].
Phototherapy bleaches the skin; hence TcB measurements in neonates undergoing phototherapy may be unreliable. Therefore, it was proposed by many investigators that TcB measurements from a site shielded from phototherapy light may reflect TCB levels with good correlation [17-22]. However, most of these studies have expressed outcome in terms of correlation [17-20]. This is a relative measure of agreement. Correlation does not provide insight into the clinical utility of a test and depends on the range of distribution of bilirubin in the study population. On the other hand, agreement between two techniques helps us to examine the degree of variation/ concordance between a new test (TcB) and the reference standard (TsB). There is only one such study which looked at agreement and demonstrated good agreement between the two measures with a mean difference of simply 0.2mg/dl 21. However, these results were from a predominant Caucasian population.
An attempt has been made in this study to find a correlation between transcutaneous and serum bilirubin levels before and after phototherapy in case of Indian babies with brownish complexion, so as to avoid number of pricks to the babies.
Materials and Methods
This prospective observational study was conducted in the Neonatal Intensive Care Unit of The Cradle Hospital at Gurgaon, Haryana, India from February 2013 to September 2013. Latepreterm (>35 weeks) and term neonates requiring phototherapy for the first time were eligible.
Inclusion criteria
i. Term and near term new born i.e. ≥35 weeks of gestational age (both intramural and extramural)
ii. Minimum birth weight 2000gm.
iii. Jaundiced newborns requiring phototherapy for first time according to the AAP guideline [16]
Exclusion criteria
i. Premature newborn (gestational age ˂35weeks)
ii. Low birth weight (<2000gms)
iii. Hemolytic Jaundice
iv. Postnatal age more than 14 days
v. Major congenital anomalies and Sick neonates
vi. Hydrops
vii. Neonates who received exchange transfusion
The bilirubin estimation was done by IL test total bilirubin which is intended for the quantitative in vitro diagnostic determination of total bilirubin in human serum and plasma using fully automated random-access biochemistry analyzer, Olympus AV -– 400. The analysis was based on modified Jendrassik -Grof assay and measurements were done within 2-3hrs of collection.
Transcutaneous measurements were performed with a twowavelength- Blue & Red (450nm, 550nm respectively) Spectral Reflectance meter (Drager Jaundice Meter- JM 103, Drager Medical Systems, USA) and operated following the manufacturer’s instructions for use.
These measurements were performed by the principal investigator or nurses on duty, trained specifically for TcB measurement in the study.
Measurements were taken over the forehead and mid sternum before starting phototherapy and after discontinuing phototherapy while the area over mid sternum was patched while the baby was on phototherapy. Averages of three readings over both the sites were recorded.
Commercially available photo-opaque patch (Bileclipse TM Phototherapy Protective patch, Murrysville, PA, USA), 2.5cm in diameter, were used to shield skin from light exposure. It was positioned over the sternum (lower one-third) prior to starting phototherapy and position check was done at 2hrly interval.
The decision to initiate phototherapy was made by the attending neonatologist using the American Academy of Pediatrics (AAP) gestation based, age-specific nomogram with risk stratification [16]. Phototherapy was given using standard phototherapy unit (Modal CFL 100, M/s Phoenix Medical systems Pvt. Ltd, India). The irradiance of the phototherapy unit was checked using a devicespecific standard flux meter (Ginevri, Rome, Italy). The neonates enrolled did not receive any drug except vitamin K which was given to all the neonates at birth.s
Continuous phototherapy was administered, and they received gavage/paladii feeding every 2 hourly under phototherapy only. As our hospital is baby friendly so always breast feeding is encouraged. So, all babies received expressed breast milk through paladii or tube (gavage feeding). To protect the eyes of the neonate, standard phototherapy eye patches were applied.
Therapy was stopped when TSB value was below the agespecific cut offs as per the AAP guidelines [16].
Considering two tailed alpha 0.05 and beta 0.20, (r=0.3), required sample size was 85, we took a sample of 100 neonates.
The primary outcome variable was the correlation between TSB and TcB after 24 hours of phototherapy on an area of patched skin spot. And also, the correlation between TSB and TcB before starting of phototherapy and post phototherapy on unpatched skin were determined. TSB, TcB (Forehead) and TcB (sternum) were recorded before initiation of phototherapy. Sternum was then shielded. After discontinuation of phototherapy, TSB, TcB (Forehead) and TcB (sternum) were recorded again.
Statistical analysis was done using graph pad prism version 5.0.
The study protocol was approved by the institution’s Ethics committee and this was registered in CTRI with the reference no. REF/2014/05/006908. Informed consent was obtained from parents.
Results
A total of 156 neonates, both intramural and extramural, had hyperbilirubinemia during the study period, out of which 138 neonates required phototherapy. 30 neonates were excluded as they did not satisfy pre-defined inclusion criteria. Out of 108 eligible neonates, who were treated with phototherapy, 8 were excluded due to feasibility issues -- no consent- (n=3), phototherapy started prior to application of patch (n=3), phototherapy started but sample for TSB not taken (n=2).
Ultimately 100 neonates were included in the study after taking into consideration, the inclusion and exclusion criteria and consent from the parents.
The mean (±SD) gestational age of the population was 37.58 (±1.121) weeks and birth weight was 2.846 (±0.454)Kg. Mean post natal age when phototherapy was prescribed was 4.43 ± 1.80 days. The median (range) age at initiation of phototherapy was 4(2-12) days. Out of 100 neonates studied 54 (54%) were males and 46 (46%) were females.
Overall mean (±SD) TcB over forehead before initiation of phototherapy was 16.22 (±2.310)mg/dl. The mean (±SD) TSB levels at initiation of phototherapy was 18.22 (±3.131)mg/dl. As all TcB (forehead) and TSB values before phototherapy passed normality test, so Pearson’s correlation coefficient was used to look for correlation. The correlation coefficient (Pearson r) between TSB and TcB (forehead) before phototherapy was 0.6152 (95% CI-0.4763 to 0.7241) and p value was <0.0001, therefore the correlation was found to be statistically highly significant.
Similarly, mean TcB (sternum) before phototherapy was 15.59 (±2.747)mg/dl. After nonpassing the normality test, correlation coefficient (Spearman r) between TSB and TcB (sternum) before phototherapy was found to be 0.6661(95% CI- 0.5362 to 0.7652) and p value was <0.0001, suggesting statistically highly significant correlation (Figure 1).
Figure 1: Correlation of post therapy forehead TcB and TSB: XY Data.
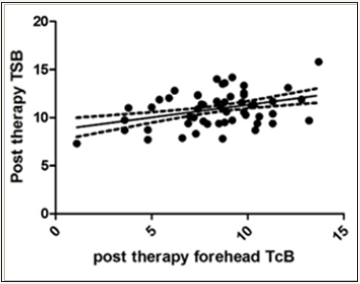
Overall mean (±SD) TcB over forehead after completion of phototherapy was 8.41 (±2.479)mg/dl. Mean (±SD) TSB levels after completion of phototherapy was 10.92 (±1.737)mg/dl. The correlation coefficient (Spearman r) between TSB and TcB (forehead) after phototherapy was 0.2747 (95% CI- 0.07689 to 0.4518) and p value <0.05 (0.0057) i.e. statistically significant.
Figure 2: Correlation of post therapy sternum TcB and TSB: XY Data.
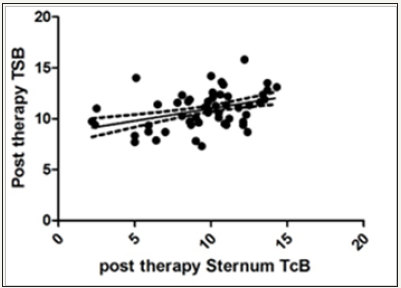
Similarly, mean TcB (sternum) (shielded site) after phototherapy was 9.67 (±2.814)mg/dl. Since TcB (sternum) values after phototherapy did not pass the normality test, therefore Spearman correlation was applied in this case between TSB and TcB (sternum) after phototherapy and was found to be 0.3959 (95% CI- 0.2106 to 0.5537), suggesting statistically highly significant correlation after Gaussian approximation of p value (<0.0001) (Figure 2).
Though both the correlation between TSB and TcB at forehead and sternum post phototherapy were significant but the correlation between TSB and TcB of the unpatched area (forehead) showed much increased number of outliers than the patched area.
Analysis of Receiver Operating characteristics (ROC) showed that post phototherapy, transcutaneous measurements at patched area (sternum) showed sensitivity of 62% (95% CI = 51.75% to 71.52%) and specificity of 52% (95% CI = 41.78% to 62.10%) with a likelihood ratio of 1.29 when TcB levels of >10.19 mg/dl were considered. Area under curve was 0.621 and P value 0.003 (Figure 3).
Figure 3: ROC of post therapy sternum TcB and TSB: ROC curve.

Discussion
In this study, there was fair correlation between TSB and TcB at shielded site (sternum) post phototherapy (r = 0.3771; 95% CI- 0.160-0.559). Also, correlation was seen between transcutaneous bilirubin at forehead and sternum and serum bilirubin both at initiation and end of phototherapy, but the correlation between TSB and TcB of the unpatched area (forehead) showed much increased number of outliers than the patched area (sternum).
Two Pevious studies [21,23] also found a correlation between plasma bilirubin levels and transcutaneous bilirubin measurements after phototherapy, using a covered area of skin. They concluded that TcB measurements through an area of covered skin could be a reliable method for use during phototherapy, reducing blood sampling. In contrast, the same correlation is not observed with transcutaneous measures taken through skin that has been exposed to phototherapy.
An overall tendency of TcB (sternum) to overestimate bilirubin was observed after phototherapy. This overall tendency of TcB (sternum) over-estimating TSB after initiation of phototherapy was also reported in a previous study [21]. The possible rationale behind the above findings seems that TcB (forehead) underestimates bilirubin levels owing to bleaching of exposed skin with phototherapy while dermal bilirubin at the shielded site, does not participate in the phototherapy induced conversion of bilirubin to its photo isomers as much as the exposed skin; thus, TcB at exposed site will underestimate bilirubin.
Transcutaneous measurements (forehead or sternum) before initiation of phototherapy showed a sensitivity of 73% (95% CI=63.20% to 81.39%) and specificity of 62 % (95% CI= 51.75% to 71.52%) when a cut off level >16.9mg/dl was considered. These observations are similar with the guidelines on neonatal jaundice commissioned by the National Institute of Health and Clinical Excellence (NICE), which states to check TSB levels in neonates with TcB more than 250 micromol/liter (14.6 mg/dl) [24].
Similarly, a sensitivity of 62% (95% CI = 51.75% to 71.52%) and specificity of 52% (95% CI = 41.78% to 62.10%) was seen post phototherapy when TcB levels of >10.1mg/dl at patched area were compared to TSB. With this degree of sensitivity and specificity it can be presumed that in cases where TcB measurements post phototherapy over patched area is <10.1mg/dl, phototherapy can be stopped reliably without the need for bloodletting for TSB measurements.
The strength of this study lies in its methodology, stringent measures taken to downplay inter-observer variation with an aim to establish the role of TcB as a surrogate marker of TSB.
References
- Anthony PJ, Stoll Barbara J (2008) Nelson Textbook of Pediatrics. In: Kliegman Robert M, et al. (eds.,). (18th edn), Saunders Elsevier publishers, New Delhi, India, pp. 756-765.
- Cloherty JP, Martin CR (2008) Mannual of Neonatal Care (6th edn). In: John CP, et al. (Eds.), Lippincot Williams & Wilkins publishers, New Delhi, India, pp.181-212.
- Dai J, Parry DM, Krahn J (1997) Transcutaneous bilirubinometry: Its role in the assessment of neonatal jaundice. Clin Biochem 30(1): 1-9.
- Lilien LD, Harris VJ, Ramamurthy RS, Pildes RS (1976) Neonatal osteomyelitis of the calcaneus: complication of heel puncture. J Pediatr 88(3): 478-480
- Bosschaart N, Joke H Kok, Newsum AM, Ouweneel DM, Mentink R (2012) Limitations and Opportunities of Transcutaneous Bilirubin Measurements. Pediatrics 129(4): 689-695.
- Rubbaltelli F, Gourley GR, Loskamp N, Modi N, Roth-Kleiner M, et al. (2001) Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics 107(6): 1264-1271.
- Ebbesen F, Rasmussen LM, Wimberley PD (2002) A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatr 91(2): 203-211.
- Lodha R, Deorari AK, Jatana V, Paul VK (2000) Non-invasive estimation of total serum bilirubin by multi-wavelength spectral reflectance in neonates. Indian Pediatr 37(7): 771-775.
- Samanta S, Tan M, Kissack C, Nayak S, Chittick R (2004) The value of BiliCheck as a screening tool for neonatal jaundice in term and nearterm babies. Acta Paediatr 93(11): 1486-1490.
- Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, et al. (2000) Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 106(2): E17.
- Dai J, Krahn J, Parry DM (1996) Clinical impact of transcutaneous bilirubinometry as adjunctive screen for hyperbilirubinemia. Clin Biochem 29(6): 581-586.
- Mishra S, Chawla D, Agarwal R, Deorari AK, Paul VK, et al. (2009) Transcutaneous bilirubinometry reduces the need for blood sampling in neonates with visible jaundice. Acta Paediatr 98(12): 1916-1919.
- Brown AH, Kim NH, Valencia G, Nuchpuchdee P, Boyle G (1984) Factors affecting the transcutaneous measurement of bilirubin: influence of the range, gestational age, phototherapy and albumin binding capacity, In: Rubaltelli FF (ed). Neonatal jaundice. New York: Plenum, USA, pp. 95- 104.
- Knudsen AA, Ebbesen F (1996) Transcutaneous bilirubinometry in neonatal intensive care units. Arch Dis Child Fetal Neonatal Ed 75(1): F53-56.
- Onks D, Silverman L, Robertson A (1993) Effects of melanin, oxyhemoglobin and bilirubin on transcutaneous bilirubinometry. Acta Paediatr 82(1): 19-21.
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia (2004) Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114(1): 297-316.
- Tan KL, Dong F (2003) Transcutaneous bilirubinometry during and after phototherapy. Acta Paediatr 92(3): 327-331.
- Hegyi T, Hiatt IM, Gertner IM, Zanni R, Tolentino T (1983) Transcutaneous bilirubinometry: dermal bilirubin kinetics during phototherapy. Paediatr Res 17(11): 888-891.
- Ozkan H, Oren H, Duman N, Duman M (2003) Dermal bilirubin kinetics during phototherapy in term neonates. Acta Paediatr 93(5): 577-581.
- Nanjundaswamy S, Petrova A, Mehta R, Hegyi T (2005) Transcutaneous Bilirubinometry in Preterm Infants Receiving Phototherapy. Am J Perinatol 2005; 22(3): 127-131.
- Zecca E, Barone G , De Luca D, Marra R , Tiberi E, et al. (2009) Skin bilirubin measurement during phototherapy in preterm and term newborn infants. Early Hum Dev 85(8): 537-540.
- Fonseca R, Kyralessa R, Malloy M, Richardson J, Jain SK (2012) Covered skin transcutaneous bilirubin estimation is comparable with serum bilirubin during and after phototherapy. J Perinat 32(2): 129-131.
- Jangaard KA, Curtis H, Goldbloom R (2006) Estimation of bilirubin using BiliChecktrade marker, a transcutaneous bilirubin measurement device: Effects of gestational age and use of phototherapy. Paediatr Child Health 2006; 11(2): 79-83.
- National Institute for Health and Clinical Excellence (2010) Health Commissioned Neonatal jaundice - National Collaborating Centre for Women’s Children’s Health Commissioned by the National Institute for Health and Clinical Excellence.
© 2018 Dr Raktima Chakrabarti. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






