- Submissions

Full Text
Research in Pediatrics & Neonatology
Development of Pericardial Calcification with Cortical Nephrocalcinosis in a Preterm Infant: A Case Report
Parth Sheth* and Darshan Shah
Department of Pediatrics, East Tennessee State University, USA
*Corresponding author: Parth Sheth, Department of Pediatrics, East Tennessee State University James H. Quillen College of Medicine, USA, Tel: 423-404- 5149; Email: shethp@etsu.edu
Submission: December 20, 2017;Published: May 04, 2018

ISSN: 2576-9200 Volume2 Issue1
Abstract
Background: Cortical nephrocalcinosis is a rare form of nephrocalcinosis in affected premature infants. Calcified pericardium with cortical nephrocalcinosis in a premature and very low birth weight infant is an unusual finding of metastatic calcification.
Case Presentation: We report the case of a 23 week 600g premature female infant with cortical nephrocalcinosis with an unusual metastatic calcification of the pericardium. The infant was born to a multigravida mother and was initially admitted for very low gestational age and birth weight, prematurity, and respiratory distress. The patient developed severe renal failure and other severe complications during the duration of intensive care of 126 days.
Conclusion: The etiologies of neonatal cortical nephrocalcinosis are numerous. The most contributory reason for developing nephrocalcinosis in premature infants is an underdeveloped renal tubular system. Calcified pericardium with cortical nephrocalcinosis is an uncommon manifestation of metastatic calcification in a premature infant. It is important to find and to manage the underlying cause for the calcification before other major organs are involved.
Introduction
Nephrocalcinosis, also known as Albright’s calcinosis or Anderson-Carr kidneys, is defined as diffuse renal parenchymal calcification that begins as fine granulations over the renal parenchyma. These granular matter eventually coalesce to form denser and more solid deposits with medullary involvement being more common than cortical [1,2]. The stone formation and findings of nephrocalcinosis are due to an imbalance between stone producing and stone inhibiting factors [1,2]. Factors contributing to the imbalance may include medications such as long term loop diuretics use for brochopulmonary dysplasia and pathologies such as hyperparathyroidism, renal tubular acidosis, or end stage renal failure [2].
Nephrocalcinosis is more frequently observed incidentally in premature neonates. The incidence in neonates is 7-41% in recent case reports [1-5]. The most likely reasoning is because of the immaturity of the renal tubular system. A neonate’s renal system’s maturity is also indirectly correlated with birth weight in which lower birth weight infants have poor renal system maturity. In fact, full maturity of the neonatal renal system is not reached until 34 to 36 weeks gestation [6]. In addition, very low birth weight infants have decreased reabsorption capacity for bicarbonate, which alkalinizes the urine pH and enhancing calcium stone precipitation [1-5]. Preterm infants also have lower glomerular filtration rates because of lower plasma flow rate through the tubular system. This process promotes stone production because of oversaturating the tubular fluid and worsening by hypocitraturia and hypercalciuria [1-7].
Other factors may also contribute to nephrocalcinosis in neonates by inducing hypercalciuria and hypercalcemia. Although rare in presentation, subcutaneous fat necrosis develops nephrocalcinosis from inflammation of the subcutaneous layer [8]. This process often follows from birth trauma, therapeutic cooling, or meconium aspiration [8].
Genetic conditions such as Williams-Beuren syndrome from hemizygous deletion of sequences on chromosome 7q11.23, idiopathic infantile hypercalcemia from mutations in CYP24A1 gene encoding 25-hydroxyvitamin D3-24-hydroxylase, and congential lactase deficiency also cause nephrocalcinosis [9-11]. Finally, nutrition such as Vitamin D therapy and total parentreral nutrition cause hypercalcemia and nephrocalcinosis [1-6].
Most nephrocalcinosis findings in neonates are found incidentally by imaging. The most common methods to detect it are ultrasound, radiography, and computed tomography, which reveal calcium deposits within the renal parenchyma accompanied by variable compromised renal function [12-15]. These imaging modalities also reveal extrarenal manifestations of the underlying cause of the nephrocalcinosis with the most common being nearby retroperitoneal involvement, gastrointestinal tract, and the lungs.
In this report, we describe one case of cortical nephrocalcinosis in a premature infant with calcified pericardium due to a pathologic process called metastatic calcification.
Case Presentation
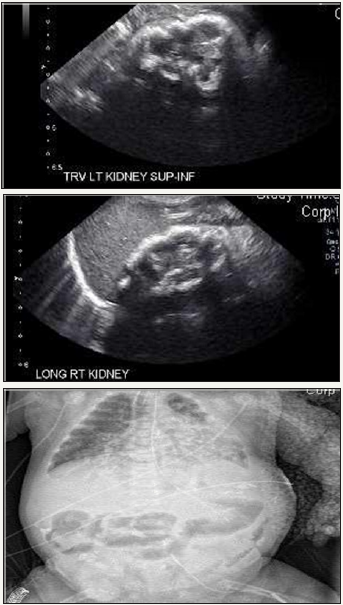
A 23.1 week 600g female infant was born as a dichorionic diamnionic twin to a 34 year old multigravida African American mother with history of neonatal demise, preterm labor, cervical dilatation and incompetent cervix via emergent Cesarean section for prolapse of body parts in the vaginal canal. The prenatal labs, blood work, and parental drug history were within normal limits. The infant was initially managed for respiratory distress and received routine care for a premature infant. The infant required intensive care for 126 days for worsening renal failure, nephrocalcinosis, severe bronchopulmonary dysplasia, metabolic acidosis, cystic periventricular leukomalacia, diastolic heart failure, and hypothyroidism. The patient’s nephrocalcinosis was present in the setting of severe renal failure and complicated by hypothyroidism, hyperparathyroidism, hypercalcemia, and hyperphosphatemia. Cortical nephrocalcinosis was detected and confirmed by imaging. Imaging revealed calcium deposits within the renal parenchyma and pericardium, indicating nephrocalcinosis and extrarenal calcification of the pericardium (Figure 1).
Figure 1: Calcifications visible in the kidneys and cardiac silhouette. Images reveal cortical nephrocalcinosis and calcification of cardiac silhouette. (a) Ultrasound transverse left kidney. (b) Ultrasound longitudinal right kidney. (c) X-ray.
Urine output and metabolites were closely monitored, and the patient was taking furosemide for the hyperphosphatemia and hypercalcemia. Neonatal profiles were taken regularly and the patient’s main source of nutrition was total parenteral nutrition through portions of the intensive care course. Patient received intensive care for a total of 126 days.
Discussion
Nephrocalcinosis in neonates is most often observed in preterm infants for a variety of reasons. The most likely reason is the renal system not reaching full maturity, which is at 34-36 weeks gestation. Nephrocalcinosis is a process that develops from an inbalance of stone producing and stone inhibiting factors. It presents initially as fine granular deposits within the renal parenchyma with undetectable or no clinical symptoms. However, these deposits may become denser and accumulate within major portions of the renal system: cortical, medullary, or mixed.
The most common form of macroscopic nephrocalcinosis is medullary (97% of cases) versus cortical (2%) and mixed (1% of cases) [13]. Premature infants have lower glomerular filtration and lower renal plasma flow rates than term infants. Neonates of lower birth weights, especially premature infants, tend to have a lower capacity to reabsorb bicarbonate than appropriate birth weight and term infants. Inevitably, these factors lead to greater stone production. Other major reasons that contribute to nephrocalcinosis may primary hyperparathyroidism, persistent renal tubular acidosis, medullary sponge kidney, hyperoxaluria (primary, enteric, or toxic), secondary to neoplasm, and drugs (furosemide and amphotericin B) [1-6,12]. Other reasons may include subacute fat necrosis, nutritional reasons (high Vitamin D or TPN), or genetic causes [8-11].
An important method of detecting nephrocalcinosis is by imaging. The best methods are by ultrasonagraphy, radiography, or computed tomography [12-15]. Axial computed tomography without contrast is considered the best method for differentiating macroscopic nephrocalcinosis and localizing calcifications [12-15]. Neonatal nephrocalcinosis can still be adequately identified via ultrasound or other radiographic methods [12-15].
Most extrarenal findings in the presence of nephrocalcinosis are retroperitoneal structures surrounding the kidneys and the peritoneum [12-15]. Severe persistence of the calcification process that led to the nephrocalcinosis has the potential of affecting other major organs such as the heart as seen in our case. Identifying and appropriately managing the root cause of the nephrocalcinosis are vital for the management of the patient.
Conclusion
The etiologies of neonatal cortical nephrocalcinosis are numerous. The most contributory reason for developing nephrocalcinosis in premature infants is an underdeveloped renal tubular system. Calcified pericardium with cortical nephrocalcinosis in a premature infant as a result of metastatic calcification is a rare finding. Overall, it is important to find and to manage the underlying cause for the calcification before other major organs are involved.
Informed Consent
Informed consent was obtained from all individual participants included in the publication of this case report and any accompanying images. A copy of the written consent is available for review by the author, DS.
Compliance with Ethical Standards
This article does not contain any studies with animals performed by any of the authors. This article is in compliance with the East Tennessee State University/Johnson City Medical Center Ethics and Research Committee. Informed consent was obtained from all individual participants included in the publication.
Authors’ Contribution
PS coordinated the ascertainment of information on the patient and wrote the manuscript. DS was the patient’s treating physician providing all of the information on the patient along with reporting the case to the appropriate agencies. All authors participated in the final adjustments of the manuscript.
References
- Schell-Feith EA, Kist-van Holthe JE, Conneman N, van Zwieten PH, Holscher HC, et al. (2000) Etiology of nephrocalcinosis in preterm neonates: association of nutritional intake and urinary parameters. Kidney Int 58(5): 2102-2110.
- Hoppe B, Duran I, Martin A, Kribs A, Benz-Bohm G, et al. (2002) Nephrocalcinosis in preterm infants: a single center experience. Pediatr Nephrol 17(4): 264-268.
- Narendra A, White MP, Rolton HA, Alloub ZI, Wilkinson G, et al. (2001) Nephrocalcinosis in preterm babies. Arch Dis Child Fetal Neonatal Ed 85(3): F207-213.
- Hein G, Richter D, Manz F, Weitzel D, Kalhoff H (2004) Development of nephrocalcinosis in very low birth weight infants. Pediatr Nephrol 19(6): 616-620.
- Schell-Feith EA, Kist-van Holthe JE, van Zwieten PH, Zonderland HM, Holscher HC, et al. (2003) Preterm neonates with nephrocalcinosis: natural course and renal function. Pediatr Nephrol 18(11): 1102-1108.
- Schell-Feith EA, Kist-van Holthe JE, van der Heijden AJ (2010) Nephrocalcinosis in preterm neonates. Pediatr Nephrol 25(2): 221-230.
- Habbig S, Beck BB, Hoppe B (2011) Nephrocalcinosis and urolithiasis in children. Kidney Int 80(12): 1278-1291.
- Shumer DE, Thaker V, Taylor GA, Wassner AJ (2014) Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed 99(5): F419-421.
- Schlingmann KP, Ruminska J, Kaufmann M, Dursun I, Patti M, et al. (2016) Autosomal-Recessive Mutations in SLC34A1 Encoding Sodium-Phosphate Cotransporter 2A Cause Idiopathic Infantile Hypercalcemia. J Am Soc Nephrol 27(2): 604-614.
- Figueres ML, Linglart A, Bienaime F, Allain-Launay E, Roussey-Kessler G, et al. (2015) Kidney function and influence of sunlight exposure in patients with impaired 24-hydroxylation of vitamin D due to CYP24A1 mutations. Am J Kidney Dis 65(1): 122-126.
- Saarela T, SimiläS, Koivisto M (1995) Hypercalcemia and nephrocalcinosis in patients with congenital lactase deficiency. J Pediatr 127(6): 920-923.
- Karunarathne S, Udayakumara Y, Govindapala D, Fernando H (2012) Medullary nephrocalcinosis, distal renal tubular acidosis and polycythaemia in a patient with nephrotic syndrome. BMC Nephrol 13: 66.
- Serra A, Correia M (2004) [Human medullary nephrocalcinosis]. Rev Port Nefrol Hipert 18: 15-32.
- Zanuto AC, Bueno T, Delfino VD, Mocelin AJ (2012) Nephrocalcinosis in a patient with Sjögren’s syndrome/systemic lupus erythematosus. Rev Assoc Med Bras 58: 279-280.
- Semins MJ, Lang E, Matlaga BR (2009) Nephrocalcinosis. J Urol 182(6): 2910-2911.
© 2018 Parth Sheth. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






