- Submissions

Full Text
Research & Investigations in Sports Medicine
Time Course Changes in Muscle Size and Echo Intensity Following Resistance Training
Eric J Sobolewski*, Keely A Simmons and Brooke A Spears
Molnar Human Performance Laboratory, Health Science Department, Furman University, USA
*Corresponding author:Eric J Sobolewski, Molnar Human Performance Laboratory, Health Science Department, Furman University, USA
Submission: February 07, 2024;Published: February 23, 2024

ISSN: 2577-1914 Volume10 Issue2
Abstract
Following resistance training, muscles swell due to a combination of damage, an increased demand for oxygen, and buildup of substrates. Cross-Sectional Area (CSA), thickness, and Echo Intensity (EI) are measurements that have been used to monitor muscle damage and recovery. Due to the variety in responses to resistance training, the aim of this study was to examine the time course changes in muscle size and EI of the elbow flexors and knee extensors following an acute bout of resistance training and determine the potential differences in response between males and females. Twenty Males (71.00±2.07cm, 74.11±9.37kg, Age 19.85±0.41) and 20 females (63.35±2.99cm, 60.62±8.92kg, Age 20.00±0.72) had panoramic ultrasound images taken of the largest circumference of the Bicep Bracii (BB), Vastus Medialis Oblique (VMO) and Vastus Lateralis (VL) and were used to determine CSA, muscle thickness and EI. Participants performed four sets to failure at 70% on a bicep curl and leg extension machine with a 90 second rest between sets. Ultrasounds were done pre and post exercise, 24 and 48-hours post. Males and females had similar responses over time (P=0.17-0.96) for each variable. Post exercise values for CSA, thickness, and EI (P<0.001) where all large but returned to baseline values by 24hrs. Males had large muscle size (P<0.001), and lower EI values (P<0.001) of the knee extensors but not of the elbow flexors (P=0.769). This study demonstrated that immediately following resistance training, muscle size and EI were significantly larger for both males and females and returned to baseline in 24hrs. Males and females exhibited similar patterns; however, males had larger knee extensor and elbow flexors muscles and lower EI values of the knee extensors on average than females.
Keywords:Fatigue; Muscle swelling; Muscle quality; Ultrasound imaging
Introduction
Acute muscle swelling or transient hypertrophy is a common phenomenon that happens during exercise. Swelling has been attributed to an increase in blood flow to the muscle because of an increase in metabolic demand by the muscle [1]. To measure acute muscle swelling researchers have used Ultrasound (US) images to measure changes in muscle size via muscle thickness measures [1,2] and Cross-Sectional Area (CSA) [3]. These measurements have both been shown to be valid when compared to CT [4] and MRI [5] scans, and reliable [6] amongst different clinicians. Muscle thickness has been shown to increase following resistance training [7], concentric [2], eccentric [8] and isometric [9] contractions, however it is not conclusive as others have shown size not to increase following resistance training [10]. However, since these studies have used single images to measure muscle thickness, it has not been determined if panoramic images [9] are better at measuring muscle size changes than single US images. Therefore, it has not been determined if muscle swelling is better measured via muscle thickness or CSA. Another component of a US image is the use of grey scale analysis, commonly referred to as Echo Intensity (EI]. This analysis uses the pixels of the image to determine the muscle quality with 0 being a pure black pixel and 255 being a pure white pixel [11]. The darker the pixels of the muscle the more structural and contractual proteins commonly referred to as quality muscle [12] The whiter the pixels, the more fibrous the tissue [13,14], intramuscular adiposity [15,16], glycogen stored [17] and water content [18].
Furthermore, EI is being used as an indicator of muscle damage [19] as it has shown to increase with both concentric [7], eccentric [20], isometric [9] exercise as well as plyometric training [21]. Most of the research evaluating EI as a marker of muscle damage is a result of resistance exercises, yet EI has not shown to change with endurance resistance type exercise [20]. It is theorized that immediate EI changes may be a result of increased blood and water content rather than muscle damage [1]. However, a study by Radaelli et al [7] found that there were no changes in EI following 4 sets of 10 repetitions at 80% max of the elbow flexors. They did find that 24 hours following the bout of exercise EI did increase. It is still unclear if EI is effect following an acute bout of concentric work since other studies [2,10] have found no difference. Addressing the full body of literature in the analysis of muscle swelling and/or muscle damage following an acute bout of exercise, it is still unclear whether muscle thickness or CSA is more sensitive to acute muscle swelling, and if EI is immediately affected or is an indicator of muscle damage. It is also interesting to note that majority of the previous research in this field focuses on only one sex, females [7] or males [2], thus is it unknown if sex plays a role in the acute responses to resistance training. Therefore, the purpose of this study was to evaluate the effect of a single bout of exercise on muscle size and EI immediately following, and a subsequent 48-hour period. A secondary aim was to determine if there are sex and image type differences when evaluating the effects of resistance training.
Methods
Participants
Twenty Males (71.00±2.07cm, 74.11±9.37kg, Age 19.85±0.41 years) and 20 females (63.35±2.99cm, 60.62±8.92kg, Age 20.00±0.72 years) volunteered for the study. Participants were familiar with resistance training as they self-reported performing this type of training 2-6 hours per week (4.4-hour average). Prior to any testing participants read and signed an informed consent and answered a health history questionnaire. All participants were free of any neurological disease or musculoskeletal injuries. This study was approved by the Institutional Review Board for the protection of human participants in accordance with the Helsinki Declaration.
Protocol
Each participant visited the lab on four separate occasions. The first visit was a familiarization day in which each participant became familiar with the leg extension machine (Precor, Woodinville, WA, USA) and bicep curl machine (Cybex, Life Fitness, Franklin Park, I, USA). They then performed a single limb max effort lift on each machine. Their second visit was the first testing day, which consisted of an ultrasound assessment followed by three sets of failure of the single leg extension and single arm bicep curls on the same machines used for testing at 70% of their 1RM. Following the sets to failure the ultrasound assessment was performed again. 24 and 48 hours post exercise subject had another ultrasound assessment performed.
Ultrasound assessment
US images were taken with a portable B-mode imaging device (GE Logic e BT12, GE Healthcare, Milwaukee, WI, USA) and a multifrequency linear-array probe (12 L-RS, 5-13 MHz, 38.4mm field of view, GE Healthcare, Milwaukee, WI, USA). The panoramic function was used to obtain images of the right Vastus Medialis Oblique (VMO), Vastus Lateralis (VL) and the Bicep Bracii (BB) in the transverse plane. Images of the VMO measurements were taken at the point of largest circumference of the muscle. Images of the VL were taken at 3/4 of the distance between the anterior superior iliac spine and the superior border of the patella. A high-density foam pad was secured around the right thigh with an adjustable strap. Images of the BB were taken on the largest circumference of the bicep Bracii. A Velcro strap was used to ensure probe movement in the transverse plane. US settings (Frequency: 10MHz, Gain: 45dB, Dynamic Range: 72) were kept consistent across participants. To ensure optimal image clarity, scanning depth was individualized for each participant between 3.5-6.0cm. A generous amount of watersoluble transmission gel (Aquasonic 100 ultrasound transmission gel, Parker Laboratories, Inc., Fairfield, NJ, USA) was applied to the skin so that it immersed the probe surface during testing to enhance acoustic coupling. Consistent with the work of Young et al. [10], three images were taken for each participant and the mean of these values used for analysis. Skin was marked so that following the exercise protocol to ensure measurements were taken at the exact same spot. The US images were digitized and examined with ImageJ Software (version 1.46, National Institutes of Health, Bethesda, MD, USA). The polygon function was used to outline the border of the RF and VL and then both the EI and CSA were measured and assessed with a computer-aided grey-scale analysis using the histogram function. The EI values are determined as the corresponding index of muscle quality ranging between 0 and 255 A.U. (black=zero, white=255).
Statistical analysis
For all variables a 20-participant reliability study was conducted in addition to the current methods. The reliability is specific to the researcher who conducted this research [6]. The statistics of interest were Intra-class Correlation Coefficients 3.1 (ICCs), Coefficient of variation, the Standard Error of Measurement (SEM), and the Minimum Difference (MD) values. Test- retest reliability data were analyzed using a custom-written software program (Microsoft Excel, Microsoft Corporation, Redmond, WA, USA). Systematic variability for each variable across testing days was examined using separate one-way repeated-measures ANOVAs [22]. A Factorial (sex) Repeated Measure ANOVA was run comparing Pre/Post/24hrs/48hrs for Muscle CSA, MT and EI for the VMO, VL, and BB. Follow up Bonferroni corrected paired sample t-tests were used to compare across time if significance was found. All statistical analyses were performed using Statistical Package for Social Science (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY). Alpha levels set a priori at P≤0.05 to determine statistical significance for all statistical analysis.
Result
Cross section area (Figure 1)
Vastus lateralis: Reliability, ICC3.1=0.989, F=0.107, P=0.746, SEM=0.5218, MD=1.446. Result of the ANOVA indicated no interaction effect (F=0.426, P=0.735). A main affect for time (F=7.946, P<0.001) and a main effect for Sex (F=16.207, P<0.001). T-test indicated that post CSA was larger than all other time points (P<0.001) with all other time points being similar (P>0.05).
Figure 1:VL=Vastus Lateralis, VMO=Vastus Medialis Oblique, BB=Bicep Brachii, * Denotes significant difference between POST and all other time points, # Denotes that males have significantly large Cross Section Area the females across all muscles.
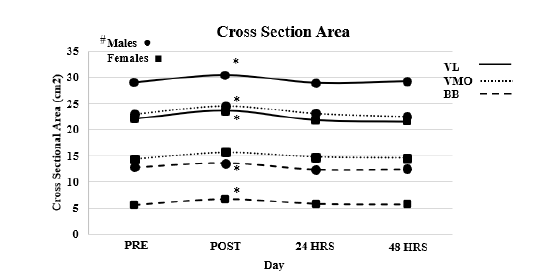
Vastus medialis oblique: Reliability, ICC3.1=0.994, F=0.061, P=0.809, SEM=0.326, MD= 0.905. Results of the ANOVA indicated no interaction effect (F=0.995, P=0.399). A main affect for time (F=14.054, P<0.001) and a main effect for Sex (F=45.093, P<0.001). T-test indicated that post CSA was larger than all other time points (P<0.003) with all other time points being similar (P>0.05).
Bicep bracii
Reliability, ICC3.1=0.995, F=0.025, P=0.621, SEM=0.178, MD=0.492. Results of the ANOVA indicated no interaction effect (F=1.685, P=0.174). A main affect for time (F=26.483, P<0.001) and a main effect for Sex (F=48.663, P<0.001). T-test indicated that post CSA was larger than all other time points (P<0.001) with all other time points being similar (P>0.05).
Echo intensity (Figure 2)
Vastus lateralis: Reliability, ICC3.1=0.988, F=0.128, P=0.723, SEM=1.144, MD=3.171. Results of the ANOVA indicated no interaction effect (F=1.444, P=0.234). A main affect for time (F=3.889, P=0.011) and a main effect for Sex (F=44.152, P<0.001). Follow up T-test did not indicate a significant difference (P>0.05) between time points.
Figure 2:VL=Vastus Lateralis, VMO=Vastus Medialis Oblique, BB=Bicep Brachii, * Denotes significant difference between POST and all other time points for BB, # Denotes that males have significantly less Echo Intensity the females for VL am VMO. ^ Denotes POST significantly different from PRE only.
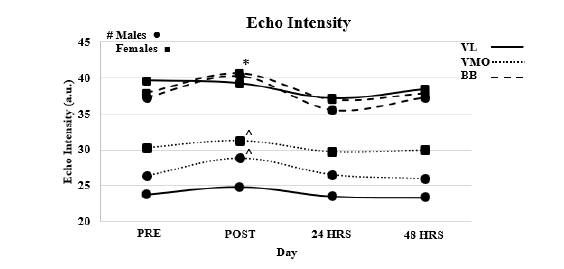
Vastus medialis oblique: Reliability, ICC3.1=0.955, F=0.148, P=0.705, SEM=1.378, MD= 3.819. Results of the ANOVA indicated no interaction effect (F=3.486, P=0.078). A main affect for time (F=3.328, P=0.022) and a main effect for Sex (F=4.882, P=0.033). T-test indicated that post EI was larger than pre (P<0.014) with all other time points being similar (P>0.05).
Bicep bracii: Reliability, ICC3.1=0.990, F=0.002, P=0.669, SEM=0.619, MD=1.718. Results of the ANOVA indicated no interaction effect (F=0.101, P=0.966). A main affect for time (F=5.434, P=0.002) and no main effect for Sex (F=0.087., P=0.769). T-test indicated that post EI was larger than pre (P<0.017) with all other time points being similar (P>0.05).
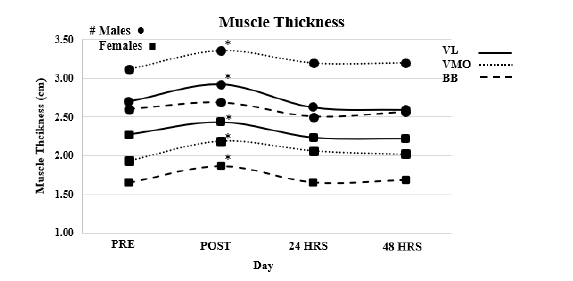
Muscle thickness (Figure 3)
Vastus lateralis: Reliability, ICC3.1=0.988, F=0.488, P=0.493, SEM=0.036, MD=0.102. Results of the ANOVA indicated no interaction effect (F=1.297, P=0.279). A main affect for time (F=35.513, P<0.001) and a main effect for Sex (F=13.271, P=0.001). T-test indicated that post MT was larger than all other time points (P<0.004) with all other time points being similar (P>0.05).
Vastus medialis oblique: Reliability, ICC3.1=0.997, F=1.266, P=0.274, SEM=0.037, MD= 0.101. Results of the ANOVA indicated no interaction effect (F=0.207, P=0.891). A main affect for time (F =17.805, P<0.001) and a main effect for Sex (F=74.549, P<0.001). T-test indicated that post MT was larger than all other time points (P<0.004) with all other time points being similar (P>0.05).
Bicep bracii: Reliability, ICC3.1=0.996, F=0.209, P=0.653, SEM=0.037, MD=0.105. Results of the ANOVA indicated no interaction effect (F=1.146, P=0.383). A main affect for time (F=111.512, P<0.001) and a main effect for Sex (F=74.549, P<0.001). T-test indicated that post MT was larger than all other time points (P<0.002) with all other time points being similar (P>0.05) (Figure 3).
Figure 3:VL=Vastus Lateralis, VMO=Vastus Medialis Oblique, BB=Bicep Brachii, * Denotes significant difference between POST and all other time points. # Denotes that males have significantly large Muscle Thickness the females across all muscles.
Discussion
This is the first study to evaluate if CSA, MT and EI are affected by resistance training to fatigue and how long those effects last. The results of this study demonstrated that CSA, MT and EI all increase following resistance training to fatigue but return to normal within 24 hours. A secondary finding was that males and females responded like resistance training as both showed similar patterns in increased CSA, MT and EI however males had larger muscles in general [23]. A third finding was that CSA and MT have similar patterns in their response to resistance training [24]. The increase in CSA and MT can be attributed to an increase in muscle swelling because of an increase in metabolic demand [25]. This increase in metabolic demand increases plasma enzyme levels [26], edema [1] and buildup of metabolites [25]. These changes however are acute in nature as US images at 24 and 48 hours did not show any changes from baseline in CSA or MT. As far as whether CSA or MT is a better indicator of muscle swelling, both showed similar results and followed similar patterns. Based on the results from this study CSA and MT can both be used to measure changes in muscle size following exercise. Conclusions on EI can be drawn like CSA and MT that the contribution to EI is a result of muscle swelling [19] as it pertaining to an increase in blood flow [1]. EI was speculated to be a marker of muscle damage as Radaelli et al [7] found that EI did not increase immediately after exercise but 24 hours post. We did not measure any changes in EI for any muscle at the 24-hour time point. This could be attributed to the load 70% vs 80% or the volume three set to failure vs 4x10. Both protocols were designed to elicit muscle failure and muscle damage, however, no indication of muscle damage was present in our study as 24 or 48 hours post measurements returned to baseline values.
Taking into consideration the results of this study and results of previous literature, it can be concluded that not all exercise types elicit the same acute EI and CSA responses; as concentric [7], eccentric [21], and cycling [27] workouts increase CSA, but not EI, while isometrics [9] elicit increase in both EI and CSA immediately following exercise. The physiological response may be highly dependent on the type of exercise, intensity and duration. Thus, it may be hard to determine the exact underlying mechanism that led to these changes. A limitation to this current study is that there were no other measurements of muscular performance other than ultrasound images. Other studies have used EMG [8] and peak torque [7] and blood markers [28] as a measurement of fatigue, muscle damage and performance. Future studies are needed to build on this research and previous literature to determine what contributes to changes in US images and how they differ between types of exercise. In conclusion, three sets to failure at 70% max elicited increases in CSA, MT and EI immediately following exercise but there were no affects seen 24 and 48 hours post, with both females and males exhibiting the similar results. CSA and MT were both in agreement and can be used interchangeably to address acute changes in muscle size. Changes in EI can be primarily attributed to an increase in blood flow and did not indicate muscle damage [17]. Caution is advised when using the US to detect acute changes and muscle damage as results may be exercise and tasks specific.
References
- Damas F, Phillips SM, Lixandrão ME, Vechin FC, Libardi CA, et al. (2016) Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol 116(1): 49-56.
- Stock MS, Oranchuk DJ, Burton AM, Phan DC (2020) Age, sex and region-specific differences in skeletal muscle size and quality. Appl Physiol Nutr Metab 45(11): 1253-1260.
- Ahtiainen JP, Hoffren M, Hulmi JJ, Pietikäinen M, Mero AA, et al. (2010) Panoramic ultrasonography is a valid method to measure changes in skeletal muscle cross-sectional area. Eur J Appl Physiol 108(2): 273-279.
- Noorkoiv M, Nosaka K, Blazevich AJ (2010) Assessment of quadriceps muscle cross-sectional area by ultrasound extended-field-of-view imaging. Eur J Appl Physiol 109(4): 631-639.
- Minnehan KS, Dexter WW, Holt CT, Scharnetzki L, Alex JP, et al. (2022) Validation of panoramic ultrasound measurement of the cross-sectional area of the vastus medialis. J Strength Cond Res 37(1): 41-45.
- Stock MS, Mota JA, DeFranco RN, Grue KA, Jacobo A, et al. (2017) The time course of short-term hypertrophy in the absence of eccentric muscle damage. Eur J Appl Physiol 117(5): 989-1004.
- Reimers K, Reimers CD, Wagner S, Paetzke I, Pongratz DE (1993) Skeletal muscle sonography: A correlative study of echogenicity and morphology. J Ultrasound Med 12(2): 73-77.
- Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19(1): 231-240.
- Muddle TW, Magrini MA, Colquhoun RJ, Luera MJ, Tomko PM (2019) Impact of fatiguing, submaximal high-vs. low-torque isometric exercise on acute muscle swelling and echo intensity in resistance-trained men. J Strength Cond Res 33(4): 1007-1019.
- Young H, Jenkins NT, Zhao Q, Mccully KK (2015) Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve 52(6): 963-971.
- Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, et al. (2009) Skeletal muscle ultrasound: Correlation between fibrous tissue and echo intensity. 35(3): 443-446.
- Mayans D, Cartwright MS, Walker FO (2012) Neuromuscular ultrasonography: Quantifying muscle and nerve measurements. Phys Med Rehabil Clin N Am 23(1): 133-148.
- Arts IM, Schelhaas HJ, Verrijp KC, Zwarts MJ, Overeem S, et al. (2012) Intramuscular fibrous tissue determines muscle echo intensity in amyotrophic lateral sclerosis. Muscle Nerve 45(3): 449-450.
- Ploutz Snyder LL, Convertino VA, Dudley GA (1995) Resistance exercise-induced fluid shifts: Change in active muscle size and plasma volume. 269: R536-R543.
- Richardson RS, Frank LR, Haseler LJ (1998) Dynamic knee-extensor and cycle exercise: Functional MRI of muscular activity. Int J Sports Med 19(3): 182-187.
- Yu JY, Jeong JG, Lee BH (2015) Evaluation of muscle damage using ultrasound imaging. J Phys Ther Sci 27(2): 531-534.
- Hill JC, Millan IS (2014) Validation of musculoskeletal ultrasound to assess and quantify muscle glycogen content: A novel approach. Phys Sportsmed 42(3): 45-52.
- Sobolewski EJ, Wein LD, Crow JM, Carpenter KM (2021) Intra-rater and inter-rater reliability of the process of obtaining cross-sectional area and echo intensity measurements of muscles from ultrasound images. J Ultrason 21(84): 7-11.
- McKay BD, Yeo NM, Jenkins ND, Miramonti AA, Cramer JT (2017) Exertional rhabdomyolysis in a 21-year-old healthy woman: A case report. 31(5): 1403-1410.
- Fritsch CG, Dornelles MP, Severo-Silveira L, Marques VB, Rosso I De A, et al. (2016) Effects of low-level laser therapy applied before or after plyometric exercise on muscle damage markers: Randomized, double-blind, placebo-controlled trial. Lasers Med Sci 31(9): 1935-1942.
- Nosaka K, Newton M, Sacco P (2002) Muscle damage and soreness after endurance exercise of the elbow flexors. Med Sci Sports Exerc 34(6): 920-927.
- Yitzchaki N, Kuehne TE, Mouser JG, Buckner SL (2019) Can changes in echo intensity be used to detect the presence of acute muscle swelling? Physiol Meas 40(4): 045002.
- Walker S, Davis L, Avela J, Häkkinen K (2012) Neuromuscular fatigue during dynamic maximal strength and hypertrophic resistance loadings. J Electromyogr Kinesiol 22(3): 356-362.
- Radaelli R, Bottaro M, Wilhelm EN, Wagner DR, Pinto RS (2012) Time course of strength and echo intensity recovery after resistance exercise in women. J Strength Cond Res 26(9): 2577-2584.
- Sarvazyan A, Tatarinov A, Sarvazyan N (2005) Ultrasonic assessment of tissue hydration status. Ultrasonics 43(8): 661-671.
- Ahmadizad S, El-Sayed MS (2005) The acute effects of resistance exercise on the main determinants of blood rheology. J Sports Sci 23(3): 243-249.
- Eric Sobolewski, Gabrielle CR, Andrew BH, Morgan OR (2020) The effect of aerobic exercise on quadriceps echo intensity and cross-sectional area. Physiology 2: 6-9.
- Morán NR, Pérez CE, Mora RR, De Cruz SE, González Badillo JJ, et al. (2017) Time course of recovery following resistance training leading or not to failure. Eur J Appl Physiol 117(12): 2387-2399.
© 2024 Eric J Sobolewski. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






