- Submissions

Full Text
Research & Investigations in Sports Medicine
Spinopelvic Parameters Following Central Decompressive Laminoplasty in Patients with Degenerative Lumbar Spinal Canal Stenosis
Mohammed Khalid Saleh*, Mohamad H Fahmy and Yamen Safwat
Department of Orthopedic Surgery, Zagazig University, Egypt
*Corresponding author:Mohammed Khalid Saleh, Department of Orthopedic Surgery, Zagazig University, Egypt
Submission: October 02, 2023;Published: October 11, 2023

ISSN: 2577-1914 Volume10 Issue1
Abstract
Background data: Central Decompressive Laminoplasty (CDL) is a minimally invasive lumbar surgery that provides a wide decompression of neural components while maintaining the spine’s stability and minimal resection of critical osteoligamentous structures. Purpose: The present study aims to assess the outcome and long-term results of CDL in the treatment of patients with degenerative lumbar canal stenosis regarding post-operative spinopelvic parameters, symptom relief, postoperative back pain, and wound complications. Study design: Patient Sample, Outcome Measures: A retrospective study was conducted over 30 patients and carried out at Zagazig university Hospital, Spine units, Orthopedic department, from October 2019 to May 2021. Patients and methods: Pre-operative radiological assessment was done using standing lumbosacral X-rays (anteroposterior, lateral, flexion and extension views), MRI, and/or CT, with obtaining following parameters using the Surgimap computer program; Spino-pelvic parameters using X-ray, AP Dural sac diameter (mm), Dural Sac Cross-Sectional Area (DSCSA) (mm2). Clinical evaluation was done with (VAS), and the Oswestry Disability Index (ODI). Result: The study showed no significant change in spinal stability, the mean pre-operative PT, PI, SS and LL were 20.81±6.58°, 49.31±12.54°, 29.37±4.32° and 35.44±12.36° respectively, while the post-operative PT, PI, SS, and LL were 21.10±5.79°, 51.30±11.69°, 30.86±5.81° and 37.69±9.82° respectively. The study showed improvement of VAS from 8.16±0.71 to 1.20±0.41 showing 84.5% improvement, while VAS of low back pain improved from 4.33±1.07 to 1.75±0.62 showing 59.5% improvement. The mean anteroposterior thecal diameter improved from 6.54±1.2mm to 12.79±1.6mm with 95.6% improvement. Conclusion: CDL is a safe, effective, less invasive, and easily applied surgical decompressive technique for treating degenerative LSS. The technique does not affect the spinopelvic parameters negatively.
Keywords:Lumbar canal stenosis; Low back pain; Outcomes; Degenerative; Treatment; Laminoplasty; Minimal invasive; Decompression
Abbreviations:LSS: Lumbar Spinal Stenosis; C7PL: The Seventh Cervical Vertebral Plumb Line; CDL: Central Decompressive Laminoplasty; VAS: Visual Analog Scale; ODI: Oswestry Disability Index; PT: Pelvic Tilt; PI: Pelvic Incidence; SS: Sacral Slope
Introduction
The population age and prevalence of degenerative spinal disorders increased the number of spinal fusion procedures. Spinal degenerative disorders include spinal canal stenosis, foraminal stenosis, degenerative scoliosis, and spondylolisthesis [1]. Lumbar Spinal Stenosis (LSS) has been classified into congenital and acquired LSS. Congenital LSS includes idiopathic and achondroplasty LSS and acquired LSS including degenerative, post-traumatic, combined, spondylolisthesis, iatrogenic and miscellaneous [2]. The main aim of surgical treatment is to widen the spinal canal and is kept for selected patients with severe and unrelieved symptoms [3]. The exact management of LSS is still undetermined, with diversity and debate highlighted by an interactive survey at the Euro spine Congress 2014 [4]. Classically, open laminectomy was the standard method of treatment for LSS, although increasingly other modalities are being utilized like laminotomy. Both techniques aim to ameliorate neural structure compression, neurologic claudication, and/or severe radicular symptoms [5]. Laminectomy is accompanied by excessive blood loss, higher postoperative wound pain, longer hospital stays, larger paraspinal muscle affection, and risk of iatrogenic segmental vertebral instability with a high incidence of pseudoarthrosis which is treated with instrumented fusion [6-10].
Decompressive laminectomy is considered the main technique, which includes the total excision of the posterior ligaments and minimal undercutting of the facet joints (Figure 1A). It provides a wide operative window which in turn supplies adequate decompression of all anatomical zones. This technique is associated with a high incidence of nonunion about 27 to 30% affecting the vertebral stability and sagittal profiles explained by the presence of a very small area of the transverse processes and the facet joints preserved for fusion after the decompression [11,12]. The seventh Cervical Vertebral Plumb Line (C7PL) showed posterior migration together with increased Lumbar Lordosis (LL) after decompressive laminectomy. These sagittal profiles may be improved due to pain alleviation and increased function after decompressive laminectomy [13]. Central Decompressive Laminoplasty (CDL) is a minimally invasive decompressive lumbar surgery that provides a wide decompression of neural components while maintaining the stability of the spine and minimal resection of critical osteoligamentous structures [14]. The present study aims to assess CDL’s outcome and long-term results in treating patients with degenerative lumbar canal stenosis regarding postoperative spinopelvic parameters, symptom relief, postoperative back pain, and wound complications. Our hypothesis is that central decompressive laminoplasty as a minimal invasive decompressive technique in management of lumbar canal stenosis is safe and effective and preserving the spinopelvic parameters (Figure 1B).
Fgure 1A:Preoperative sagittal MRI.

Fgure 1B:Post-operative sagittal MRI.

Material and Methods
Patient demographic
Ethical Review and Study Design After approval by the Zagazig University institutional review board ethical committee (ZU-IRB# 10524-29-3-2023), 30 patients were selected to be included in this study between October 2019 and May 2021.Informed consent was obtained from all patients (Figure 1C).
Fgure 1C:Preoperative axial MRI.

Inclusion criteria: 1) Adult patients diagnosed with degenerative lumbar canal stenosis who didn’t respond to at least six months of non-operative treatment regimen 2) Degenerative lumbar pathologies associated with severe leg with/without back pain with sagittal lumbar canal AP diameter less than 11mm.
Exclusion criteria included: Patients with traumatic or spondylotic lumbar spine fractures and/or previous traumatic spinal instability, revision cases, patients with pure lateral stenosis, and congenital stenosis, patients who are medically unfit for anesthesia, and those who refused to participate were excluded also (Figure 1D).
Fgure 1D:Post-operative sagittal MRI.

Pre-operative evaluation
The patients of the study were diagnosed by complete history, clinical examination, and radiological assessment. Pre-operative radiological assessment was done using standing lumbosacral X-rays (anteroposterior, lateral, flexion, and extension views), MRI and/or CT, with obtaining following parameters using the Surgimap computer program; Spino-pelvic parameters using X-ray, AP dural sac diameter (mm), Dural Sac Cross-Sectional Area (DSCSA) (mm2). Visual Analog Scale (VAS) for both leg and back pain was assessed preoperatively, with 0 equals no pain and 10 meaning maximum. The patient quality of life was measured using the Oswestry Disability Index (ODI).
Operative techniques
The 3rd generation of Cephalosporin one hour pre-operatively is given intravenously for all patients. Under general anesthesia, the patients are catheterized and placed in the prone position with the hips flexed to reduce normal lumbar lordosis and increase the interlaminar space, making it easier to access the lumbar canal. Using an image intensifier, the operative level is verified. A midline skin incision is made over the involved level with incision length depending on the number of levels to be operated. The thoracolumbar fascia is incised with a cautery knife; The interlaminar space and the bony details were exposed by subperiosteal separation of the paraspinal muscles. Use Sponges to separate the paraspinal muscles laterally and avoid bleeding by heavily packing the opened space between the muscle and the spinous process. Resecting the lower part of the cephalad lamina and to a small extent, from the superior edge of the caudal lamina, then we do the same to the contralateral side.
Resecting the medial part of the facet joint to expand the lateral recess. The ligamentum flavum and its bony attachments are completely excised to expose and decompress the Dural sac. Undercutting and trimming of the underlying surface of both laminae and the spinous process is done using Kerrison rongeur. The nerve roots on both sides are confirmed to be completely decompressed (Figure 1 E&F). The spinous process, the supraand interspinous ligaments, and a considerable percentage of the lamina stayed conserved. Hemostasis is confirmed with the use of bipolar electrocautery and hemostatic agents such as an absorbable gelatin sponge (Gel foam) if needed. A suction drain is placed in the deep wound.
Fgure 1E&F:Intra operative photo showing decompression with preserving midline structures.

Post-operative management
The suction drain was extracted when it measures less than 50ml in 6 hours. All patients were imaged radiologically on day one after surgery and then discharged from the hospital starting from the second day after the operation.
Follow up
VAS and ODI were collected along with plain x rays at 6 weeks, 3 months, and every 3 months till 2 years. After 1 month postoperative all patients had MRI and/or CT. The radiological union was determined in the last follow-up by radiographies and CT scans to detect bone healing in between the fused vertebrae.
Statistical analysis
The sample size was measured with the consideration that the central decompressive laminoplasty technique may result in a 50% increase in the anteroposterior diameter of the lumbar canal. The measured sample sizes ranged from 20 to 28 patients, and we approximated the highest calculated number of 28 to be 30 patients. The measures of multiple values (preoperative, and one year after surgery) were compared. All statistical analyses were performed with SPSS version 24.0. P<0.05 was considered statistically significant (Figure 2).
Fgure 2:Post-operative 3 D CT showing preserved midline structures.

Result
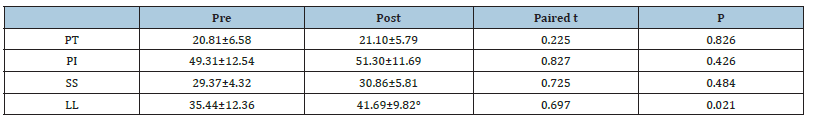
The study was performed on 30 patients (24 females and 6 males) with an average age of 54.50±6.59 (range (46-67) years and BMI of 39.34±4.67 (Table 1). The total number of operated levels was 47 levels; the majority were in L4-L5 & L5&S1. The single-level procedure was performed in 13 patients (41.7%) and a doublelevel procedure in 17 patients (58.3%). L4-5 level was operated on in 8 patients, L5-S1 in 7 patients, L3-4 in 5 patients, and L2-3 in 5 patients (Table 2). The mean Incision length was distributed as 7.83±1.33 cm, while the mean Estimated Operative Blood Loss (EBL) was 275.16±50.89ml (Figure 3). The mean operative duration was 95.75±11.7 minutes and fusion are done in 2 cases (Table 3). The VAS of leg pain changed from 8.16±0.71 to 1.20±0.41 at the last follow-up with 84.5% improvement, while s the VAS of the back pain showed 59.5% improvement as it changed from 4.33±1.07 to 1.75±0.62 at the last follow up (6 months). The ODI changed from 79.25±12.16 to 6.78±2.98 with a 91.1% improvement at the last follow-up (P<0.001) (Table 4). The mean anteroposterior thecal diameter improved from 6.54±1.2mm to 12.79±1.6mm at the last follow-up with 95.6% percent of improvement (P<0.001), while the mean DSCSA changed from 60.34±6.8mm2 to 110.65±4.82mm2 with 83.3% improvement at the last follow up (P<0.001) (Table 5 & 6). The mean LL of the studied group was 35.44±12.36° at baseline, which increased significantly to 41.69±9.82° at the 1-year followup (P=0.021) however other spine pelvic parameters showed no significant change as follows; The mean pre-operative Pelvic Tilt (PT), Pelvic Incidence (PI), Sacral Slope (SS), were 20.81±6.58°, 49.31±12.54°, 29.37±4.32° respectively, while the profundities PT, PI, SS, were 21.10±5.79°, 51.30±11.69°, 30.86±5.81° respectively with no significant change founded (Table 7), (Figure 4). No cases of intra-operative Dural tears had occurred in our cases. One patient was presented with a superficial wound infection and received antibiotic injections and the infection subsided through repeated dressing.
Table 1:Basic demographic and clinical data distribution among studied group (N=12).
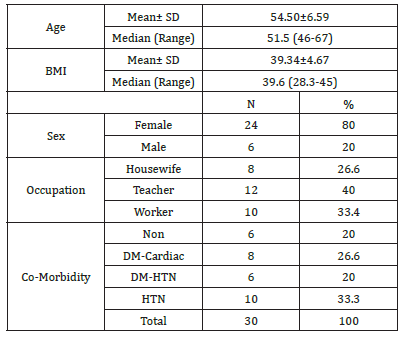
Table 2:Number and distribution of stenotic levels.
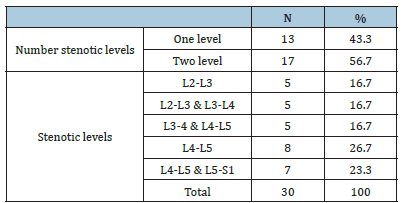
Table 3:Operative data distribution among studied group.
Fgure 3:Preoperative and post-operative x ray with spino pelvic parameter measurements.

Table 4:VAS and ODI distribution among studied group at different times of follow up.
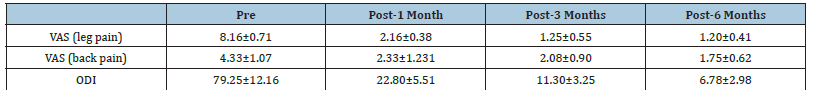
Table 5:Dural sac cross sectional area distribution among studied group at pre and post at each level.
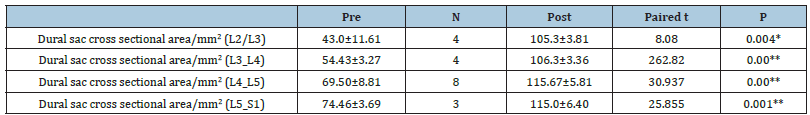
Table 6:Spinal canal pre-and post-operative mean AP diameters among studied group at pre and post at each level.
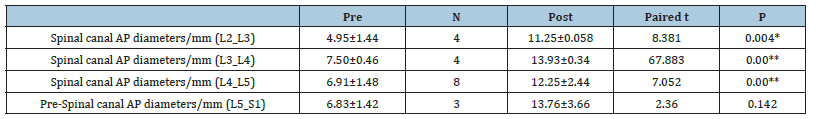
Table 7:Pre-and post-operative mean spinopelvic parameters among studied group at each level.
Fgure 4:Bar graphs showing pre-and post-operative data of: A) spinal canal AP diameter, B) DSCSA, C) Spinopelvic parameters.

Discussion
Global sagittal balance, spinopelvic morphology, and sagittal alignment should be considered as important factors in surgical planning. The spinopelvic morphology affects the lumbosacral configuration and consequently, the mechanical forces at the lumbosacral junction [15-22]. In their study, most of the patients had neurologic claudication and the prevalence of sagittal imbalance in patients with lumbar canal stenosis was 31.2% which was significantly higher compared to controls [15-22]. Over the last few years, Different surgical procedures have been described for lumbar spine decompression. Symptomatic relief is the main surgical goal of treatment which is achieved by adequate neural decompression while preserving osteoligamentous anatomy as much as possible, thus preserving the biomechanical function of the lumbar spine [16]. North American Spine Society (NASS) guidelines suggest the use of decompressive surgery as a means of improving outcomes not only in patients with severe symptoms of LSS but in those with moderate symptoms as well [17]. Ramhmdani et al. reported that of 105 patients with decompression in the form of laminectomies across 1-4 levels, 10 patients (9.5%; 5 male and 5 female with an average age of 63.0±11.2 years) complicated with iatrogenic spondylolisthesis at the same operated levels that required another surgery [18].
Nasca RJ has recommended that an arthrodesis can be performed when lumbar stenosis is associated with spinal instability or in the setting of a spondylotic spine associated with low back pain [19]. The mean age was 53.50±6.59 years old compared to other studies, the mean age was 64.4 (range, 46-87) years [14] and 47.7±10.4 (30- 60) years [20]. The mean LL of the studied group was 35.44±12.36° at baseline, which increased significantly to 41.69±9.82° at the 1-year follow-up (P=0.021) this may be explained by improvement in patient symptoms and decreased postoperative pain; However other spino pelvic parameters showed no significant change as follow; The mean pre-operative PT, PI, SS, were 20.81±6.58°, 49.31±12.54°, 29.37±4.32°respectively, while the post-operative PT, PI, SS, were 21.10±5.79°, 51.30±11.69°, 30.86±5.81° respectively with no significant change founded. Suzuki et al. [21] studied the sagittal parameters in patients with Lumbar Canal Stenosis (LCS) (N=93, mean age 66.8 yrs.). The cases were divided into 2 groups: The claudicate type and the nerve root type. The mean pelvic tilting angle of the claudicate type LCS (27.2°±8.3°) was greater than that of the nerve root-type LCS (22.7°±7.2°). The mean lumbar lordotic angle of claudicate-type LCS (18.8°±13.2°) was smaller than that of the nerve root-type LCS (22.4°±14.0°), [22]. The spinopelvic parameters including the mean SS and LL were significantly lower in the LCS group than the non-LCS group as shown in the study done by Abbas et al. [22] comparing both groups using reconstructed CT scans [23].
The mean spino sacral angle, PI, SS, PT, and TK had not significantly changed after decompressive laminectomy at the 1-year follow-up (P=0.069, 0.225, 0.400, 0.195 and 0.062 respectively) or at the 2-year follow-up (P = 0.071, 0.751, 0.773, 0.126 and 0.072, respectively) [24]; however the LL of laminectomy group was significantly increased from 31.4°±15.1° to 35.6°±11.7° at 1 year follow up and that was preserved till 2 years follow up, and the C7PL showed posterior migration in the same group [24]. The incidence of post-laminectomy kyphosis was presented in 6% to 47% of adults and up to 100% of children as reported in a recent meta-analysis. The mean anteroposterior thecal diameter changed from 6.54±1.2mm to 12.79±1.6mm at the last follow-up with 95.6% percent of improvement (P<0.001), while the mean DSCSA improved from 60.34±6.8mm2 to 110.65±4.82mm2 showing 83.3% improvement at the last follow up (P<0.001). Hermansen et al. [20] study showed improvement of the mean DSCSA from 80mm2 preoperatively to 161mm Postoperatively with an increase of 81mm2 (101%). A study by Saleh et al. [19] showed improvements in the mean thecal sac AP diameter from 10.4±1.4 (Range, 6-13) mm to 14.1±1.1 (Range, 12-16)mm at the last follow-up with 35.6% percent of improvement (P<0.001), while the thecal sac crosssectional area improved from 134.2±19.6 (Range, 110-170)mm2 to 184±20.4 (Range, 150-220)mm2 showing 37.1% improvement at the last follow up (P<0.001). The mean Estimated Operative Blood Loss (EBL) was 264.16±50.89ml which was less than that of the Liu et al. [3] study which was 291.1mL for sublaminartrimming laminoplasty alone, 657.7mL for sublaminar-trimming laminoplasty with PLF, also it was less than Peddada et al. [1] study which was 600ml. The mean operative time was distributed as 93.75±11.7 with a minimum of 80 and maximum of 115 minutes which was less than and less than Liu et al. [3] which was126.6min for sublaminar trimming laminoplasty alone, also it was less than Saleh et al. [19] study in which the mean operative time was 127.5±35.3 (Range, 85-200) minutes.
The VAS of leg pain improved from 8.16±0.71 to 1.20±0.41 at the last follow up showing 84.5% improvement, while the VAS -of the back pain showed 59.5% improvement as it changed from 4.33±1.07 to 1.75±0.62 at the last follow up (24 months). Compared to other studies, Kwon et al. study showed that the mean VAS scores of back/ buttock pains improved post-operatively (from 5.1±2.0 to 1.3±2.6) with an improvement of 75%, while the VAS for leg pain improved from 5.5±2.1 pre-operatively to 1.5±2.2 post-operative showing 76.2% improvement. Liu et al. [3] study showed a decrease of preoperative VAS for leg pain and back pain from 6.8±1.3 and 6.1±1.2 respectively into 0.6±1.1 and 1.8±1.0 post-operative showing 91.1% and 70% improvement respectively at the last follow up (3 years). The ODI improved from 79.25±12.16 to 6.78±2.98 showing a 91.1% improvement at the last follow-up (P<0.001), compared to Kwon et al. study that showed a decrease of ODI from 42.8±18.1 to 19.0±21.6, (p<0.001) with 55.6% improvement at the last follow up (6 months), and also in comparison to Liu et al. [3] study that showed improvement of ODI from 30.8±5.8 to 9.4±5.9 with 69.4% improvement at the last follow up (3 years). The presented study has some limitations including the small number of the study group and the absence of the control group and retrospective nature of the study. Future prospective studies that include a greater number of participants with randomization are needed. This could be achieved by a well-randomized multicenter study including a control group.
Conclusion
CDL is a safe, effective alternative to laminectomy and an easily applied surgical option for treating degenerative LSS that allows both great field visualization and satisfactory central and lateral decompression of the neural elements with insignificant resection of critical bony structures, and insignificant change to spinopelvic parameters.
- Peddada K, Elder BD, Ishida W, Lo SL, Goodwin CR, et al. (2016) Clinical outcomes following sublaminar decompression and instrumented fusion for lumbar degenerative spinal pathology. J Clin Neurosci 30: 98-104.
- Ekman P, Moller H, Tullberg T, Neumann P, Hedlund R (2007) Posterior lumbar interbody fusion versus posterolateral fusion in adult isthmic spondylolisthesis. Spine (Phila Pa 1976) 32(20): 2178-2183.
- Liu WJ, Lu T, Hong SW, Liou DY (2014) Clinical outcomes following sublaminar-trimming laminoplasty for extensive lumbar canal stenosis. Eur Spine 23(1): 80-86.
- Fornari M, Robertson SC, Pereira P, Zileli M, Anania CD, et al. (2020) Conservative treatment and percutaneous pain relief techniques in patients with lumbar spinal stenosis: WFNS spine committee recommendations. World Neurosurg X 7: 100079.
- De Kunder SL, Rijkers K, Van Hemert WLW, Willems PCPH, Ter Laak-Poort MP, et al. (2016) Transforaminal versus posterior lumbar Interbody fusion as operative treatment of lumbar spondylolisthesis, a retrospective case series. Interdisciplinary Neurosurgery 5: 64-68.
- Costa F, Anania CD, Zileli M, Servadei F, Fornari M (2020) Lumbar spinal stenosis: Introduction to the World Federation of Neurosurgical Societies (WFNS) spine committee recommendations. World Neurosurg X 7: 100075.
- Ye YP, Xu H, Chen D (2003) Comparison between posterior lumbar interbody fusion and posterolateral fusion with transpedicular screw fixation for isthmic spondylolisthesis: A meta-analysis. Archives of Orthopaedic and Trauma Surgery 133(12): 1649-1655.
- DiPaola CP, Molinari RW (2008) Posterior lumbar interbody fusion. J Am Acad Orthop Surg 16(3): 130-139.
- Kebaish KM, Elder BD, Lo SL, Witham TF (2017) Sublaminar decompression: A new technique for spinal canal decompression in the treatment of stenosis in degenerative spinal conditions. Clin Spine Surg 30(1): 14-9.
- Boden SD, Schimandle JH, Hutton WC (1995) An experimental lumbar intertransverse process spinal fusion model: Radiographic, histologic and biomechanical healing characteristics. Spine 20: 412-420.
- Hasegawa K, Kitahara K, Shimoda H, Hara T (2013) Biomechanical evaluation of destabilization following minimally invasive decompression for lumbar spinal canal stenosis. J Neurosurg Spine 18(5): 504-510.
- Thomas NW, Rea GL, Pikul BK, Mervis LJ, Irsik R, et al. (1997) Quantitative outcome and radiographic comparisons between laminectomy and laminotomy in the treatment of acquired lumbar stenosis. Neurosurgery 41(3): 567-574.
- Deer T, Sayed D, Michels J, Josephson Y, Li S (2019) A review of lumbar spinal stenosis with intermittent neurogenic claudication: Disease and diagnosis. Pain Med 20(2): S32-S44.
- Barry C, Jund J, Noseda O, Roussouly P (2007) Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases: A comparative study about 85 cases. Eur Spine J 16(9): 1459-1467.
- Nemani VM, Aichmair A, Taher F, Lebl DR, Hughes AP, et al. (2014) Rate of revision surgery after stand-alone lateral lumbar interbody fusion for lumbar spinal stenosis. Spine 39(5): E326-31.
- Nakai O, Ookawa A YI (1991) Long-term roentgenographic and functional changes in patients who were treated with wide fenestration for central lumbar stenosis. J Bone Jt Surg Am 73(8): 1184-1191.
- Kabil M, Ebrahim K (2017) Micro endoscopic bilateral decompression via unilateral approach in single and multiple level lumbar canal stenosis: A series of 583 cases. Egy Spine J 25: 6-16.
- Abouelmaaty E, El Molla S (2017) Assessment of clinical outcome of bilateral decompression through unilateral approach in lumbar canal stenosis. Egy Spine J 21: 24-32.
- Saleh MK, Abdelrazek MA, Shamel Elgawhary (2018) Sublaminar decompression and fusion in the management of stenotic lumbar degenerative disorders. Egy Spine J 28(1): 32-39.
- Hermansen, Tor Åge Myklebust, Ivar Magne Austevoll (2019) Clinical outcome after surgery for lumbar spinal stenosis in patients with insignificant lower extremity pain. A prospective cohort study from the Norwegian registry for spine surgery. BMC Musculoskelet Disord 20(1): 36.
- Suzuki H, Endo K, Kobayashi H (2010) Total sagittal spinal alignment in patients with lumbar canal stenosis accompanied by intermittent claudication. Spine 35(9): E344 -346.
- Abbas J, Hamoud K, May H, Hay O, Medlej B, et al. (2010) Degenerative lumbar spinal stenosis and lumbar spine configuration. Eur Spine J 19(11): 1865-1873.
- Jeon CH, Park JU, Chung NS, Son KH, Lee YS, et al. (2013) Degenerative retrolisthesis: Is it a compensatory mechanism for sagittal imbalance? Bone Joint J 95B(9): 1244 -1249.
- Jun Zhang, Tang-Fen Liu, Hua Shan, Zhong-Yuan Wan, Zhe Wang (2021) Decompression using minimally invasive surgery for lumbar spinal stenosis associated with degenerative spondylolisthesis: A Review. Pain Ther 10(2): 941-959.
© 2023 Mohammed Khalid Saleh*. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






