- Submissions

Full Text
Research & Development in Material Science
Effect of Diluents on The Extraction of Vanadium from Chloride Media Using Bis(2-Ethylhexyl) Phosphate
Dada Oluwashina Emmanuel1, Ayorinde Emmanuel Ajiboye2*, Ojo James Oluwasanjo1
1Department of Chemistry, Federal University of Technology, Nigeria
2Department of Chemical Engineering, University of Cape Town, South Africa
*Corresponding author:Ayorinde Emmanuel Ajiboye, Department of Chemical Engineering, University of Cape Town, South Africa
Submission: December 11, 2023;Published: January 10, 2024

ISSN: 2576-8840 Volume 19 Issue 5
Abstract
This study investigated the effect of diluents on the extraction of V(V) from hydrochloric acid solution using bis(2-ethylhexyl) phosphate as extractant. It was determined by phosphor-tungsten method of determination. The percentage extractions were found optimum at a concentration of 25% (v/v) bis(2-ethylhexyl) phosphate in n-heptane (E%=51.9) while it was found at optimum concentration of 10 % (v/v) bis(2-ethylhexyl) phosphate for both xylene (E%=36.9) and kerosene (E%=17.0). The optimum extraction temperature of V(V) occurred through an exothermic process at 301 K for all the diluents. Kerosene and xylene require the least quantity of D2EHPA (10 % v/v) compared with 25 % v/v for n-heptane and may be more economical for use. On batch extraction stages, bis(2-ethylhexyl) phosphate gave a percentage extraction by mass of V(V) (52.0%) in n-heptane, V(V) (58.6%) in xylene and V(V) (72.6%) in kerosene showing kerosene as best for continuous extraction. From the slope analysis, analytical and spectra data, the extracted complexes have been formulated as VO2R (R= union of D2EHPA).
Keywords: Diluents; Extractants; Vanadium; Metals impurities; Chloride media
Introduction
Vanadium demand has a long history of linkage to use in steel alloying and remains an irreplaceable element in steel making. For over 100 years, steel alloying has represented over 90% of demand for vanadium, more demand would be experienced soon because of its uses in batteries and some chemical industries. The VRFB is a rechargeable flow battery using vanadium ions for energy storage, mainly in longer duration (>4.0h) grid scale applications. Demand for this type of storage is primarily driven by increasing use of variable renewable energy (solar and wind) which necessitates longer duration storage batteries. The cumulative share of energy storage using VRFB will rise to 7.0% by 2030, and to nearly 20.0% by 2040. Vanadium is produced from two major sources; those obtained from by-product of iron, uranium, phosphate and the one from different form of metal residues like spent catalyst, slag or fly ashes [1]. Because of high demand, recovery of vanadium from residues obtained during ore production of other metals like rare earth elements is gaining more attention. However, solvent extraction technique has remained established hydrometallurgical process used for the recovery of metal values from industrial waste streams [2,3]. Vanadium recovery from different media using various types of extractants has been reported in the literature [3-6]. Therefore, this paper focuses on the study of varying diluents for the solvent extraction of vanadium from chloride raffinate solution after recovery of rare earth element.
Materials and Method
Reagents and chemicals
The reagents used were of analytical grade which include Hydrochloric acid (HCl), Potassium chloride (KCl), Sodium metavanadate (NaVO3), Se, As, B, V, Al and Cr, Sulphuric acid (H2SO4), Sodium tungstate dihydrate (Na2WO4.2H2O), Phosphoric acid (H3PO4) and Nitric acid (HNO3) and bis(2-ethylhexyl) phosphate (D2EHPA). They are from BDH and MECK. Exactly 0.3048g of NaVO3 and 1.8625g of KCl were weighed and dissolved in 6M HCl in 250cm3 standard flask and made up to the mark with the acid. Different concentrations of D2EHPA in the range 0.29-0.86mol dm3 were prepared by dissolving D2EHPA in appropriate volumes of n-heptane/xylene/kerosene.
Experimental procedure
Meanwhile chromium, vanadium, aluminum, and boron are substantial impurities in the fly ash leach chloride solution. For our study, the raffinate solution after rare earth element has been recovered is our principal focus. To mimic the chloride raffinate solution, simulated solution of V, Al and Cr was prepared, and the solution contains 0.01M of (NaVO3/ 0.1M KCl), Al3+ and Cr6+ and 6M HCl. For the batch extraction, 10.0cm2 of the simulated solution was taken, 10.0cm3 of the extractant 5.0% v/v D2EHPA in n-heptane was also taken from the stock solution and mixed in the separating funnel with the lid closed, placed in a hollow container, and padded with foam to enable it stand on the orbital shaker for equilibration. The shaker was set to revolve at 100rpm for seven minutes before it was then switched on, the separating funnel was removed and reclamped; cork removed and allowed to stand for about two minutes. This allowed the mixture to settle and separate into two distinct phases: the organic and the aqueous phases. The aqueous phase (usually the lower part of the mixture) was run into a measuring cylinder from the tap, volume recorded and then emptied into a 25.0cm3 sample bottle and labeled as sample “A”. The interfacial layer was removed from the organic phase left in separating funnel by running 2- 4 drops into an empty beaker. The organic layer was poured from the top of the separating funnel into a beaker, emptied into a measuring cylinder so that the volume can be recorded, and then poured again into the separating funnel. The stripping agent HNO3 for vanadium was then poured into the separating funnel (volume equal to the volume of the organic phase already in the separating funnel), corked, and the process of shaking, settling, running of the aqueous phase into a measuring cylinder were carried out, the volume was recorded and labeled as sample “B” and the final organic phase is then discarded. The process above was repeated for extractant concentrations 10.0, 15.0, 20.0, and 25.0% (v/v D2EHPA / n-hexane, xylene, and kerosene). The electronic and infrared spectra bands of the unstripped organic and stripped organic phases were recorded on the UV-visible spectrophotometer in the range 200-900nm and the Agilent Technologies infrared Spectrophotometer in the range 4,000-650cm-1.
The effect of concentration of extractant (D2EHPA) involving the use of n-heptane, xylene and kerosene diluents on vanadium (V) extraction, effects of binary and ternary mixtures of diluents on vanadium (V) extraction, number of batch extraction stages and optimum temperature required for quantitative extraction of vanadium (V) and consequently the enthalpy change and establish the distribution coefficient and percentage extraction of bis(2- ethylhexyl) phosphate for vanadium(V) extraction from chloride media at the established optimum conditions were determined.
Results and Discussion
Effect of extractant concentration on the extraction of V(V)
Figure 1:Plot of vanadium(V) extraction efficiency from 6M HCl with concentration of D2EHPA using different diluents (n-heptane, xylene, and kerosene) [V(V)] = 0.01M; Temperature= 28 ℃.
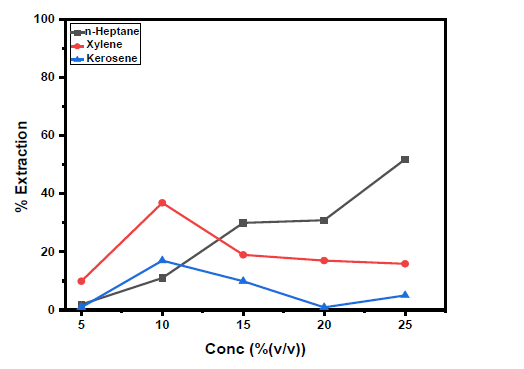
The effect of variation of concentration of extractant on extraction of V(V) from metals solution of 6M HCl solution at the optimum contact time of 7 minutes for bis(2-ethylhexyl) phosphate (D2EHPA) in different diluents (n-heptane, xylene, and kerosene) is presented in Figure 1. The concentration of extractant used in this study varied from 5 to 25.0% (v/v) of bis(2-ethylhexyl) phosphate in n-heptane, xylene, and kerosene. In n-heptane, the percentage V(V) extraction(E%) increased gradually from 1.0 to 51.9% at D2EHPA concentration of 5.0% to 25.0% v/v respectively. This trend is like that observed in the comparative study of the extraction of V(V) and V(IV) from hydrochloric acid solutions with di(2-ethylhexyl) phosphoric acid [7]. While in xylene, the E% increased to 36.9 at 10%v/v D2EHPA concentration and decreased gradually, while in kerosene, the trend was increased to 17.0 at 10%v/v D2EHPA concentration, thereafter, decreased and subsequently increased. The percentage extractions were found optimum at a concentration of 25.0% (v/v) bis(2-ethylhexyl) phosphate in n-heptane (E%=51.9) while it was found at optimum concentration of 10%(v/v) bis(2- ethylhexyl) phosphate for both xylene (E%=36.9) and kerosene (E%=17.0) respectively. The trend in xylene and kerosene might be possibly due to high interfacial tension between the aqueous and organic phases that set in at higher concentrations of the extractant above 10.0%(v/v) thereby hindering the mass transfer of solutes from the aqueous phase to the organic phase [8]. This trend is like that observed in V(V) extraction from HCl solution by D2EHPA [7]. Also, the trend is like the one observed in extraction and separation of Mo (VI) and W(V) [9]. Therefore, the extractant concentration of 25, 10 and 10%(v/v) established, was used for subsequent extraction experiments for n-heptane, xylene, and kerosene respectively. However, it is noteworthy that Cr and Al as impurities were not co-extracted at all.
Effect of binary mixture of diluents on extraction of vanadium
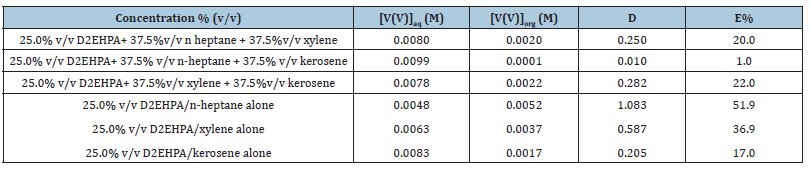
The effect of binary mixture of diluents on the extraction of V(V) was studied at the optimum extraction conditions of bis(2- ethylhexyl) phosphate by varying the diluents in three different orders; first (n-heptane and xylene), second (n-heptane and kerosene) and third (xylene and kerosene). Based on the result presented in Table 1, high extraction efficiency (E%) was observed in the first and third varying order. However, on comparing the results obtained with those of the individual diluents, it was observed that no synergistic effect was achieved, all were much lower. This, however, contradicts the study on synergistic extraction reported by some researchers [10]. This is due to high interfacial tension between the aqueous and organic phases that set in at concentration of 37.5 %v/v of the extractants thereby hindering the mass transfer of solutes from the aqueous phase to the organic phase [8].
Table 1:Effect of binary mixture of diluents on the extraction of vanadium(V) from metals solution. [V(V)] = 0.01M, KCl = 0.1M and metal solution, optimum extractant concentration: D2EHPA = 25.0% (v/v), tempt: 28 ℃.
Effect of ternary mixture of diluents on extraction of vanadium
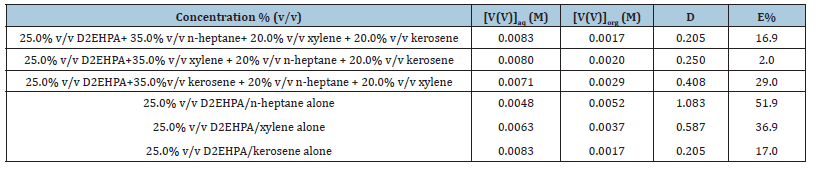
The effect of ternary mixture of diluents on the extraction of V(V) was studied at the optimum extraction conditions of bis(2- ethylhexyl) phosphate by varying the diluents in three different order 3 is presented in Table 2. From the result, it was observed that the E % was high at the first and third varying order. However, on comparing the results obtained with those of the individual diluents, it was observed that no synergistic effect was achieved (the E% was much lower).
Table 2:Effect of extractant concentration on the extraction of vanadium(V) from metals solution using ternary mixture
of diluents.
[V(V)]= 0.01M, KCl = 0.1M, optimum D2EHPA conc. = 25.0% (v/v): tempt: 28 ℃.
This is however not in tandem with the literature on the synergistic extraction reported by some researchers [10].
Effect of Temperature on the extraction of V(V)
The effect of temperature on the extraction of V(V) from metals solution of 6M HCl solution at the established optimum concentration of bis(2-ethylhexyl) phosphate is shown in Figure 2, while the plots of log D against 1/T x 103 for V(V) with bis(2-ethylhexyl) phosphate are shown in Figure 3 to Figure 5 respectively. The effect of temperature on V(V) was studied in the temperature range of 301 to 321K (28 -48 ℃). The optimum extraction temperature for the extractant was observed at 301K (28 ℃). Van’t Hoff Isotherm: The Van’t Hoff isotherm is used to determine the Gibb’s free energy for non-standard state reaction at a constant temperature and to estimate the change in enthalpy or total energy and entropy (amount of disorderliness of a chemical reaction). From Van’t Hoff equation:
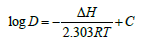
Where D and ΔH represent distribution coefficient and enthalpy change for the extractive reaction [10].
Figure 2:Variation of percentage vanadium(V) extraction from 6M HCl with temperature using different diluents (n-heptane, xylene, and kerosene), [V(V)] = 0.01M; tempt: 28 ℃.
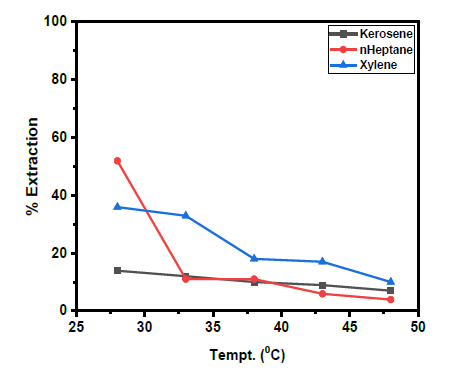
Figure 3:A plot of log D against temperature (1/T) with n-heptane as diluent [V(V)] = 0.01M, KCl = 0.1M, optimum extractant concentration: D2EHPA = 25% (v/v) in n-heptane: optimum tempt: 28 ℃.
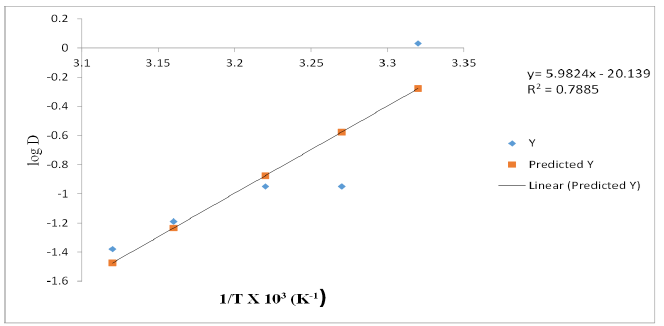
Figure 4:A plot of log D against temperature (1/T) with xylene as diluent [V(V)] = 0.01M, KCl = 0.1M, optimum extractant concentration: xylene = 10% (v/v) in n-Heptane: optimum tempt: 28 ℃.
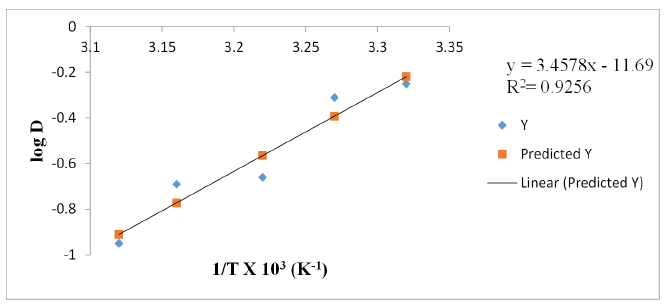
Figure 5:A plot of log D against temperature (1/T) with kerosene as diluent [V(V)]= 0.01M, KCl = 0.1M, optimum extractant concentration: kerosene = 10.0% (v/v) in n-Heptane: optimum tempt: 28 ℃.
The Van’t Hoff plot for an endothermic reaction should always be a negative slope. The reverse is for exothermic reaction.
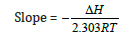
In n-heptane, the plot of log D against 1/T x 103 yielded a straight-line graph of slope equal to 5.9824 for V(V) while for the extractant in xylene and kerosene, the line graph slopes for V(V) were 3.4578 and 1.5153 respectively. The determined enthalpy change, ΔH in n-heptane was -114.5kJmol-1, xylene -66.2kJmol-1 and kerosene -29.0KJmol-1 respectively. These values indicate endothermic extractive reactions for V(V).
Effect of batch extraction stages on the extraction of V(V)
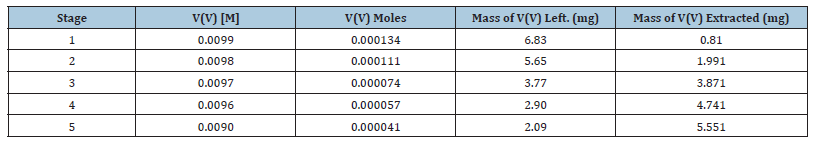
The effect of batch extraction stages on the extraction of V(V) with bis(2-ethylhexyl) phosphate in n-heptane, xylene and kerosene was studied for five batch extraction stages and the results are shown in Table 3 to Table 5 respectively. From the results shown, it was observed that the mass of V(V) extracted increased for all the diluents as the number of extraction stages increased. With the extractant dissolved in n-heptane, 0.6 and 3.9mg of V(V) were extracted (from the initial 7.641mg v(v)) at the 1st and 5th extraction stages respectively while for xylene, 0.6mg and 4.5mg were extracted for the 1st and 5th extraction stages. For kerosene, 0.8mg and 5.6mg were recorded respectively in that order.
Table 3:Effect of batch extraction stage on the mass of vanadium(V) extracted from metals solution of 6M HCl with 25.0% (v/v) D2EPHA dissolved in n-heptane.
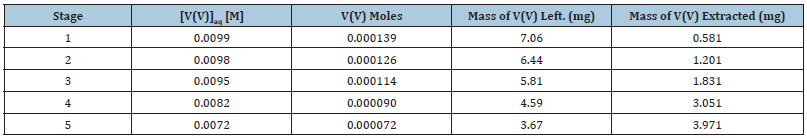
Initial mass of V(V) in 15.0cm3 of aqueous stock solution = 7.641mg, [V(V)] = 0.01M, KCl = 0.1M, optimum Extractant concentration: D2EHPA = 25.0% (v/v) in n-Heptane, tempt: 28 ℃.
Table 4:Effect of batch extraction stage on the mass of vanadium(V) extracted from metals solution of 6M HCl with 25.0% (v/v) bis(2-ethylhexyl) phosphate dissolve in xylene.
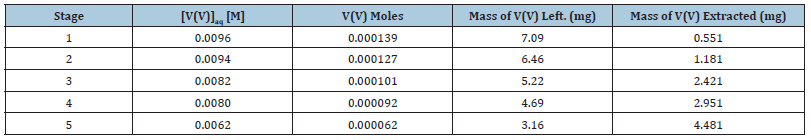
Initial mass of V(V) in 15cm3 of aqueous stock solution = 7.641mg, [V(V)]= 0.01M, KCl = 0.1M, optimum extractant concentration: D2EHPA = 10.0% (v/v) in xylene: tempt: 28 ℃.
Table 5:Effect of batch extraction stage on the mass of vanadium(V) extracted from metals solution of 6M HCl with 25% (v/v) bis(2-ethylhexyl) phosphate dissolve in kerosene.
Electronic absorption spectra data
The electronic spectra bands of V(V) with bis(2-ethylhexyl) phosphate in n-heptane, xylene and kerosene and vanadium (V) extracts are presented in Table 6. With D2EHPA/n-heptane, the electronic absorption spectra band occurs at 33,389cm-1, D2EHPA/ n-heptane extract of V(V) is 34,423cm-1 to ascribable to n→6* transition. The hypochromic or positive shift in the frequency of absorption is due to ligand-to-metal charge transfer (LMCT) [11].
Table 6:Electronic absorption spectra data for the organic phase and the extracted vanadium (V).
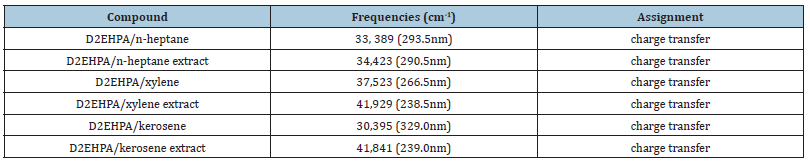
Similarly, with D2EHPA/xylene, the absorption is shown at 37,523cm-1 while D2EHPA/xylene extract is 41,929cm-1 ascribable to n→6* transition. The hypsochromic or positive shift in the frequency of absorption is due to ligand-to-metal charge transfer (LMCT) (Ojo, 2013). With D2EHPA/kerosene, the absorption is shown at 30,395cm-1 while D2EHPA/kerosene extract is shown at 41,841cm-1 ascribable to n→6* transition. The hypochromic or positive shift in the frequency of absorption is due to ligand-tometal charge transfer (LMCT) [11].
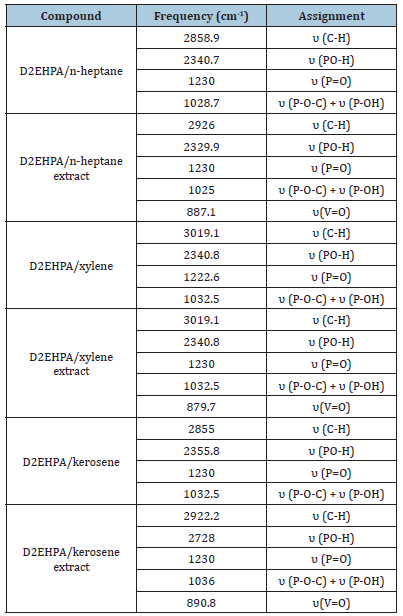
Infrared spectra studies: The infrared spectra data for V(V) in the D2EHPA/n-heptane, D2EHPA/n-heptane extract, D2EHPA/ xylene, D2EHPA/xylene extract, D2EHPA/kerosene, D2EHPA/ kerosene extract are shown in Table 7. With D2EHPA/n-heptane, it showed infrared absorption bands at 2858.9, 2340.7, 1230 and 1028.7 ascribable to υ (C-H), υ (PO-H), υ (P=O), υ (P-O-C) / υ (POH) vibrations respectively. For D2EHPA/n-heptane extract, the infrared showed absorption bands at 2926, 2329.9, 1230, 1025 and 887.1 ascribable to υ (C-H), υ (PO-H), υ (P=O), υ (P-O-C) / υ (P-OH), and υ(V=O) vibrations. Similarly, for D2EHPA/xylene, the infrared showed absorption bands at 3019.1, 2340.8, 1222.6, 1032.5 ascribable to υ (C-H), υ (PO-H), υ (P=O), υ (P-O-C) / υ (POH) vibrations respectively. For D2EHPA/n-xylene extract, the infrared showed absorption bands at 3019.1, 2340.8, 1230, 1032.5 and 879.7 ascribable to υ (C-H), υ(PO-H), υ (P=O), υ (P-O-C) / υ (P-OH) and υ(V=O) vibrations. Similarly for D2EHPA/kerosene, the infrared showed absorption bands at 2855, 2355.8, 1230, 1032.5 ascribable to υ (C-H), υ (PO-H), υ (P=O), υ (P-O-C) / υ (POH) vibrations respectively while for D2EHPA/kerosene extract, the infrared showed absorption bands at 2922.2, 2728, 1230, 1036 and 890.8 ascribable to υ (C-H), υ (PO-H), υ (P=O), υ (P-O-C) / υ (P-OH) and υ(V=O) vibrations. The differences in the absorption bands of the organic phases and their extracts show evidence of vanadium(V) extraction [12].
Table 7:Infrared absorption spectra data for the extracts of vanadium(V).
Conclusion
Recovery of vanadium (V) from simulated raffinate metals solution from rare earth element extraction was successfully studied. The result obtained shows that D2EPHA selectively recover vanadium (V) and rejected other impurities. The extraction efficiency was best using D2EPHA in kerosene followed xylene and n-heptane. It was also observed that kerosene and xylene require the least quantity of D2EHPA (10% v/v) compared with 25% v/v for n-heptane. Hence, it would be more economical to use kerosene in the event of scale up of this process. The IR-spectra data showed differences in the absorption bands of the organic phases and their extracts as evidence of vanadium(V) extraction.
Acknowledgement
The Author acknowledge the Department of Chemistry Federal University of Technology Akure Nigeria for providing the enabling environment for the success of my master’s degree.
References
- Storm (1994) Vanadium - sources, applications and markets. Minerals and Energy - Raw Materials Report10(3): 2-15.
- Toyabe KT, Kirishima KK, Shibayama HT, Hanawa HK (1995) Process for recovering valuable metal from waste catalyst, US Patent 5,431,892.
- Jayadas S, Reddy ML (2002) Solvent extraction separation of vanadium (V) from multivalent metal chloride solutions using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. J Chem Technol Biotechnol 77: 1149-1156.
- Moussa A, Habib S, Safaa A (2008) Solvent extraction of Vanadium (IV) with di(2 ethylhexyl) phosphoric acid and tributyl phosphate Chemical Engineering 52(1): 29-33.
- Li C, Wei C, Deng Z, Li M, Li X, et al. (2010) Recovery of vanadium from black shale. Trans Nonferrous Met Soc China 20(1): 127-131.
- Ojo JO, Oyegoke DA (2013) Alkanols for the extraction of vanadium(V) from hydrochloric acid solutions. International Journal of Chemistry 5(3): 123-134.
- Ojo JO, Adebayo AO, Ojo OI (2012) Comparative study of the extraction of V(V) and V(IV) from hydrochloric acid solutions with D1(2 ethylhexyl) phosphoric acid. Ife J of Sci 14(1): 15-22.
- Lofstrom-England EN (2014) On the diluent and solvent effects in liquid-liquid extraction systems based on bis-triazine-bipyridine (BTBP) ligands. Ph D Thesis Department of chemical and biological engineering. Chalmers University of Technology, Gothenburg, Sweden.
- Talla R, Gaikwad S, Pawar SD (2010) Solvent extraction and separation of Mo (VI) and W(VI) from hydrochloric acid solutions using cyanex-923 as extractant. Indian J of Chem Techn 17: 436-440.
- Kang J, Kim Y, Joo S, Yoon H, Kumar JR, et al. (2013) Behavior of extraction, stripping and separation possibilities of rhenium and molybdenum from molybdenite roasting dust leaching solution using amine based extractant tri-octyl-amine (TOA). The Japan Inst of metals and materials trans 54(7): 1209-1212.
- Ojo JO (2013) Separation of simulated mixed Mo (VI) and V(V) from nitric acid and hydrochloric acid solutions by selective extraction and stripping with tri-n-butyl phosphate as extractant. Sep Sci and Tech 48: 1577-1584.
- Lambert JB, Shurvell HF, Lightner DA, Cooks RG (1987) Macmillian Publishing Company, New York, USA, p. 217.
© 2024 Ayorinde Emmanuel Ajiboye. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






