- Submissions

Full Text
Research & Development in Material Science
Monitoring, Modeling, and Optimization of Biodesulfurization of Crude Oil by Rhodococcus erythropolis and Acidithiobacillus ferroxidans
Wisam Mohamed Kareem Al-Khazaali1 and Seyed Ahmad Ataei2*
1Chemical Engineering Sec., Designs Dept., Misan Oil Company, Iraq
2Department of Chemical Engineering, Faculty of Engineering, Shahid Bahonar University of Kerman, Iran
*Corresponding author:Seyed Ahmad Ataei, Department of Chemical Engineering, Faculty of Engineering, Shahid Bahonar University of Kerman, Kerman, Iran
Submission: October 19, 2023;Published: November 27, 2023

ISSN: 2576-8840 Volume 19 Issue 4
Abstract
Biodesalination of water is a promising method for treating the salty water due to its environmental friendliness and ability to get rid of the recalcitrant salts. In this study, two types of microorganisms Rhodococcus erythropolis (ATCC 25544, PTCC 1767) and Acidithiobacillus ferrooxidans (ATCC 23270, PTCC 1646) medium supplemented with MG- Sulfur Free Medium (SFM) and the medium PTCC 105. A monitoring for the physicochemical properties of the reaction medium has been shown. A modeling based on the monitored behaviors has been developed based on Artificial Intelligence for both microorganisms. It was found that the optimum operation conditions have been designed by using Definitive method such as mixing speed, temperature, surfactant dose, Oil Water Ration (OWR), pH. Biodesalinatio was shown in this utilization of current objective by setting the minimization of total content of salts as the main objective in the Response Surface Method (RSM). Also, a pathway optimization was designed based on the proposed model and controlling the products of bioreactions. It was found that this theoretical and applied methods can lead to a high efficiency in the treatment of oil.
Keywords: Biodesulfurization, Rhodococcus erythropolis, Acidithiobacillus ferrooxidans
Introduction
Fossil Fuel is an important source of energy or power in various fields in life and industry. Before applying it in customization use, it must be on specification of some related standards to avoid risks on HSE. Then, sulfur compounds are one of these constraints to be treated, or else, they formulate a gangerence on quality and HSE limitations heart diseases, asthma, and respiratory illnesses [1].
There are many methods of treatment such as adsorptive desulfurization-adsorption (ADS) [2], microbial and bacterial desulfurization (BDS) [3], Extraction (Desulfurization by Extraction, EDS) [4], ODS-Oxidative Desulfurization (Desulfurization by oxidation DO) [3], supercritical water-based (water) desulfurization (SWD) [5]. Also, a combination is such as BDS-ODS-EDS [6], EDS-HDT [7], OEDS (Oxidation Extraction Desulfurization) and microwave catalytic hydrogenation process [6].
BDS is more efficient and less expensive than the remaining methods as HDS in removing sulfur from refractory heterocyclic compounds present in crude oil, it could be used in oil refineries as a complement to achieve ultra-low sulfur diesel (ULSD). Indeed, BDS can be used to desulfurize heavy oils, as shale oils, which have high thiophene concentration [8].
Several studies and patents have investigated the biodesulfurization (BDS) of crude oil using various microorganisms, including Achromobacter, Leptospirillum, Pseudomonas, Sulfolobus, Thiobacillus, Rhodococcus strains, Sphingomonas subarctica, Bacillus, Desulfovibrio desulfuricans, Pyrococcus, Desulfomicrobium scambium, Desulfovibrio longreachii, and Pantoea agglomerans [9,10]. BDS of whole crude oil has the advantage of reducing the cost of desulfurization treatment in refineries [11]. Additionally, BDS can be applied to oil derivatives such as LPG, petrol, jet fuel, kerosene, fuel oil, and gas oil using various microorganisms, including Mycobacterium goodii, Pseudomonas, Gordonia, Rhodococcus, Mycobacterium phlei, Pseudomonas delafieldii, Paenibacillus, Rhodoccocus globerulus, and Nocardia [11-14]. Model compounds such as BT, DBT, DBTO2, DBTSO2, MgSO4, BNT, DBS, 2,8 DMDBT, 2,6 DNDBT, DMDBT, and thianthrene have also been used to represent the whole fossil fuel, particularly the recalcitrant HCS [8,15-24]. Finally, microorganisms used in BDS of water or coal can also be applied to oil [25].
The methodology was based on that source of sulfur is necessary for the microorganism growth, and then the objectivity can be achieved according to this behavior. The medium is set without sulfur and the oil required to be treated is the sole source of sulfur as it contain the organosulfur compounds in order to be enforced on treatment to remove.
Materials
Nutrient components: There are many chemical components required in the preparation of culture mediums of microorganisms in order to achieve the growth and desulfurization. The chemical species can be divided into carbohydrates, minerals, proteins and vitamins (ignored here), furthermore, adaptation agents, acids and bases as HCl, NaOH, and surfactants (Tween polysorbate 80 C64H124O26 and PEG as emulsification and demulsification reagents respectively.
The significant mediums were prepared by using the chemical species of medium PTCC 105 and MG-medium sulfur free mineral medium “SFM” (pH 7) as detailed by Al-Khazaali and Ataei (2023). The demulsification agents used in this study were n-heptane and polyethylene glycol (PEG), while Tween 80 was used as the pro-emulsification agent. The acidization agent used was HCl, while NaOH was used as the alkalinity agent. Rhodococcus erythropolis (PTCC 1767) was purchase from PTCC center of Tehran. Acidithiobacillus thiooxidans (PTCC 1717) was borrowed from University of Tehran.
Methods
The treated samples (oil/water) were separated using various methods. Gravity settling was employed by allowing the mixture to settle in a decanter flask for 20-30 minutes. In some cases, a chemical solvent or cleaner, such as heptane, was used as a reverse emulsifier or demulsifier. Heating the mixture at 80 C for one day was also used to separate gel, particularly in emulsion samples (No. 6, 11, 20, and 22). Centrifugation was also used to separate any remaining oil in the medium after separation, with a speed of 500rpm and time of 10 minutes being the most commonly used parameters. In some cases, centrifugation was done at 20 °C for 1 hour with a speed of 78,269g. These separation methods were reported by Chen [3].
In this study, several equipment were used for different purposes. The IKA(R) ks 4000i and HYSK Shaking Incubator, in combination with the SANYO CO2 Incubator, were utilized for incubation. Additionally, the STIK oven was employed for the evaporation and purification of the samples to prepare them for the final test. The pH and electric conductivity (EC) were measured using the Trans Instruments BP 3001 and 3020, respectively. Prior to inoculation and incubation, the mediums were sterilized using the Reuhan Teb sterilizer. The separation of water-oil phases was achieved using the centrifuge Duna Velocity 14, according to ASTM 1796. Finally, the total sulfur in the crude oil was measured using the TSN 6 200 sulfur elemental analyzer (NORDTECH) and X-Ray (in Tehran lab) was used for the same purpose.
,p>The sterilization of chemical media and laboratory equipment, such as cotton and glassware, can be achieved using an autoclave. Medium 105 was sterilized in an autoclave without FeSO4 to prevent sedimentation. FeSO4 was sterilized separately in an autoclave and then mixed with the remaining mixture, or it could be added using a micro-filter (micro syringe) with a pore size of 4μm. The sterilization conditions were set to a pressure of 15 psi and a temperature of 120 °C for 20 minutes. In the case of FeSO4, the temperature was set at 100 °C for only 5 to 10 minutes to prevent its sedimentation in the culture medium.The fermentation inoculation process took approximately two days, during which an Erlenmeyer flask containing 25-100ml of inoculum was used. The culture medium used for fermentation does not have to be the same as that used for the culture medium. For adaptation purposes, as previously mentioned, a few drops or 0.5ml of crude oil can be added. It is essential to ensure that the seed culture is inoculated for the same amount of time in all experiments to maintain a constant OD for all experiments at day two (t2). The incubator was set at a temperature of 30-35 °C, a rotation speed of 150rpm, and a time of 2 days.
The primary reaction in BDS is represented by incubation, where 50ml of medium and 5ml of the sample are treated and inoculated with 5ml of microorganisms at approximately 4% [3]. Minitab was applied to design the experiment by Definitive method and optimization of the operation conditions by Response Surface Methodology.
Results and Discussions
The study includes three parts. They are, Physicochemicalbased monitoring, AI-based modeling of BDS of crude oil, and Biodesalination-based optimization of microorganisms growth. The following subsections show these.
Physcochemical_based monitoring
The chemical compositions have been seen through measurement of both reactants and product which are contents of salts, sulfur, and microorganisms respectively. It was found a strong increase in the growth until 30hr, and then a slight increase was noted for the Rhodoccous erythropoli as shown in Figure 1(A). While, it was found that the growth of Acidithiobacillus ferroxidans was slightly in the first 20hr, then it increased more as shown in Figure 1(A).
Figure 1:The biochemical concentrations profiles [A] of reactant and product (sulfur compounds (%) [Green], salinity (mg/l*10E-3) [Blue], and microorganisms (λ) [Red]), and the chemophysical response [B] (API gravity [Green], electric conductivity [Blue], and the acidity [Red]) for the Rhodococcus erythropolis and Acidithiobacillus ferrooxidans at the average operation conditions.
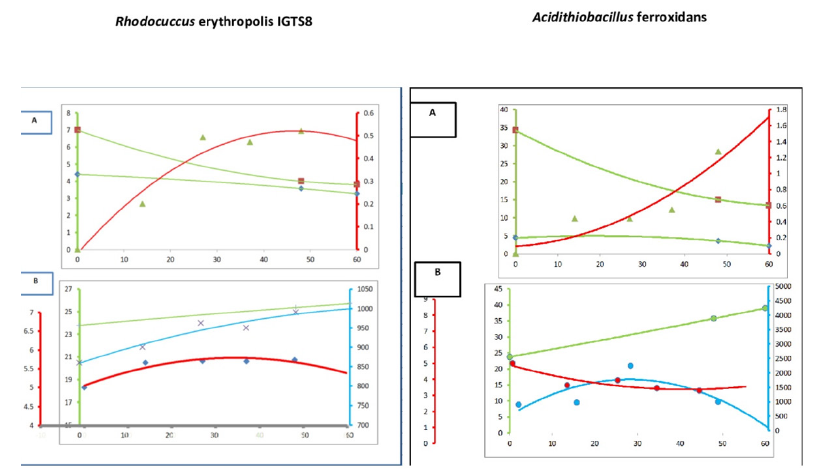
Also, the behaviors of physicochemical properties can be seen in Figure 1(B) for both microorganisms, whereas, pH values slightly increase and decrease for Rhodoccous erythropolis and Acidithiobacillus ferroxidans respectively. Therefore, the behavior of electric conductivity varies too. API increased in both microorganism for the ability on the upgrade of crude oil.
The growth of microorganisms was indicated by using the measurement of salinity represented by the conductive Total Dissolved Salts (TDS). Whereas, the microorganisms utilize these salts in the metabolism bioreactions accompanied with the sources of carbon or energy [26]. It was found that reduction of dissolved salts content present in the water as an aqueous solution. This solution formulates an environmental medium for the microorganisms because they are sources of energy, carbon, nitrogen, sulfur, trace element solution. This leads to the ability of utilization this results in supporting the idea of desalination of water by microorganisms such as Rhodococcus erythropolis, Acidithiobacillus ferrooxidans. The salts can be removed using inactivated algae biomass coated on steel mesh within a relatively short time [27,28]. After this biotreatment, a separation can be applied for removing the cells and remaining salts and nutrient sources from the desalinated water. It was found that the electric conductivity increased due to the increasing of ions by the metabolism reactions.
AI_based modeling of bds of crude oil
AI-based model by machine learning has been developed by traning the AI software (Chatgpt) to express the metabolic biochemical reaction pathway of microorganisms in the presence of sources of sulfur and both macro- and micro-nutrients salts. This was implemented by setting the metabolic pathway for Rhodococcus erythroplois to desulfurize the crude oil in SFM medium. Also, the metabolic pathway of Acidithiobacillus ferroxidans can be detected by setting 9k medium.
There can be differences in the chemical equation when the medium pH increases or decreases slightly, as changes in pH can affect the protonation state of functional groups in the enzymes involved in the metabolic pathway. This can alter the reactivity and specificity of the enzymes, leading to changes in the metabolic reactions and products. Additionally, changes in pH can also affect the solubility and availability of substrates and cofactors, which can also impact the metabolic pathway. Therefore, it is important to carefully control and monitor the pH in bioprocesses to ensure optimal conditions for the desired metabolic reactions.
The effect of slight changes in pH can be illustrated through the biochemical pathway. In general, the biochemical pathway for desulfurization of crude oil by Rhodococcus erythropolis involves several enzymatic reactions, which are influenced by the pH of the medium. The key enzymes involved in this process include sulfur-specific enzymes such as monoxygenases, desulfurases, and oxygenases.
When pH of the medium increases slightly, it can enhance the activity of these enzymes and promote the desulfurization process. For instance, the increase in pH may lead to the activation of desulfurase enzymes, which catalyze the cleavage of carbonsulfur bonds in organic sulfur compounds. This would result in the production of sulfite ions and other organic molecules such as alkanes and alkenes.
On the other hand, a decrease in pH may inhibit the activity of these enzymes and slow down the desulfurization process. For example, a decrease in pH may lead to the inhibition of oxygenase enzymes, which are responsible for oxidizing sulfur-containing compounds to form sulfones and sulfoxides. This can result in the accumulation of organic sulfur compounds in the medium, leading to a decrease in desulfurization efficiency.
Overall, slight changes in pH can affect the activity of enzymes involved in the biochemical pathway for desulfurization of crude oil by Rhodococcus erythropolis, ultimately influencing the efficiency of the process.
The formation of Rhodococcus cells involves a complex
mechanism that includes the synthesis of various enzymes and
internal cellular components. Here’s a simplified mechanism to
show the key steps involved in the formation of Rhodococcus cells:
Source of energy (Glycolysis): The glucose is broken
down through glycolysis, which yields energy and metabolic
intermediates such as pyruvate. In the citric acid cycle, pyruvate is
further oxidized through the citric acid cycle, which generates more
energy and metabolic intermediates such as NADH and FADH2.
Then, this can include Pyruvate oxidation and Citric acid cycle as
shown in Eq. 4 respectively.
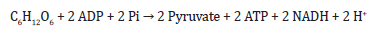
Where ADP is adenosine diphosphate, Pi is inorganic phosphate, and NAD+ and NADH are nicotinamide adenine dinucleotide and its reduced form, respectively. Citric acid cycle:
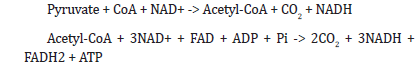
Nutrient uptake: Nutrient uptake: Rhodococcus cells take up
nutrients such as glucose, nitrogen, phosphorus, and other essential
elements from the surrounding environment to support cell growth
as shown in Eq. 1 respectively.
a) Nitrogen uptake:
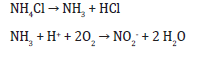
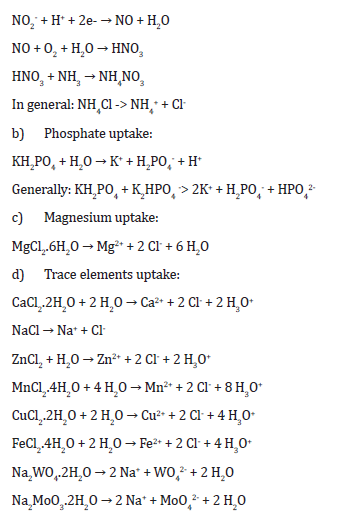
e) Glucose as a source of carbon (nutrient)
f) Sulfur removal from oil: In the case of biodesulfurization,
Rhodococcus cells also synthesize and utilize desulfurization
enzymes to remove sulfur-containing compounds from the crude
oil.
The exact pathway for sulfur removal from oil by Rhodococcus erythropolis is not well understood. However, it is thought that the microorganism uses a sulfur-specific pathway to remove sulfur from oil, such as the 4S pathway (4S pathway converts sulfurcontaining compounds into thiols) or the 3-hydroxy-2-formyl-4- methylthiobutanoic acid (HFMBA) pathway.
S-containing compound + Desulfurization enzyme -> Desulfurized compound + Reduced sulfur-containing compound
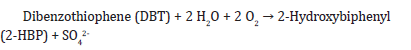
g) HCl usage: HCl can be added to the reaction mixture to adjust the pH of the medium and provide the optimum growth conditions for Rhodococcus erythropolis.
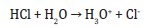
Syntheses reactions:
A- amino acids: a. Glutamate synthesis: alpha-ketoglutarate + NH4+ + NADPH + H+ → glutamate + NADP
b. Aspartate synthesis:
oxaloacetate + glutamate + ATP → aspartate + alphaketoglutarate
+ ADP + Pi c.
c. Alanine synthesis:
pyruvate + glutamate + NADH + H+ → alanine + alphaketoglutarate
+ NAD+
B- Synthesis of nucleotides:
a. Synthesis of purine nucleotides:
PRPP + glutamine + glycine + ATP → IMP + ADP + Pi + H2O + 2H+
b. Synthesis of pyrimidine nucleotides:
aspartate + carbamoyl phosphate + ATP → carbamoyl aspartate
+ ADP + Pi carbamoyl aspartate → dihydroorotate + H2O
dihydroorotate + fumarate → orotate + H2O + H+
orotate + PRPP → orotidine 5’-phosphate + PPi
Biological formations:
a) Enzyme synthesis: Enzyme synthesis: Rhodococcus cells
synthesize various enzymes required for cell metabolism, such
as synthesis of glucose transporter, phosphorus transporter,
nitrogenase, and desulfurization enzymes as shown in Eq. 2
respectively.
Amino acids + ATP → enzymes + ADP + Pi
DNA + RNA + ribosomes + amino acids -> Glucose transporter
protein
DNA + RNA + ribosomes + amino acids -> Phosphorus transporter
protein + RNA + ribosomes + amino acids -> Nitrogenase protein
DNA + RNA + ribosomes + amino acids -> Desulfurization
enzyme protein
b) Protein synthesis: Rhodococcus cells synthesize various
proteins required for cell growth and division, such as ribosomal
proteins, DNA replication enzymes, and cell wall biosynthesis
enzymes as shown in Eq. 6.
Amino acids → proteins
DNA + RNA + ribosomes + amino acids -> Protein
c) Electron transport chain: NADH and FADH2 transfer electrons to the electron transport chain, which generates a proton gradient across the cell membrane and drives ATP synthesis. This can include NADH oxidation, FADH2 oxidation, and ATP synthesis respectively as shown in Eq. 5.
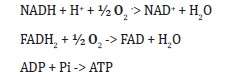
d) Cell division: Rhodococcus cells grow and accumulate sufficient internal components; they undergo cell division to produce new cells as shown in Eq. 7. Cellular components → new cells 2Cells -> 4 Cells
Other reactions:
a) Fatty acid metabolism:
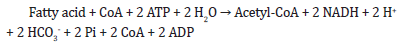
b) Amino acid metabolism:
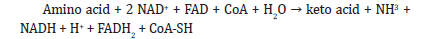
c) Pentose phosphate pathway:
Glucose-6-phosphate + NADP+ → 6-Phosphogluconate +
NADPH + H+
Overall, the mechanism of Rhodococcus cell formation involves a complex interplay between nutrient uptake, enzyme synthesis, energy generation, protein synthesis, and cell division. This process requires the coordinated regulation of multiple cellular pathways to ensure proper cell growth and division, as well as the production of specialized enzymes required for biodesulfurization.
These simplified equations represent some of the key steps involved in Rhodococcus cell formation, including nutrient uptake, enzyme synthesis, energy generation, protein synthesis, cell division, and biodesulfurization. However, it’s important to note that the actual mechanism is much more complex and involves a multitude of biochemical reactions and pathways.
The slight decrease in pH could be attributed to the production of organic acids during metabolism, while the increase in electrical conductivity could be due to the release of ions into the medium during nutrient uptake and metabolism.
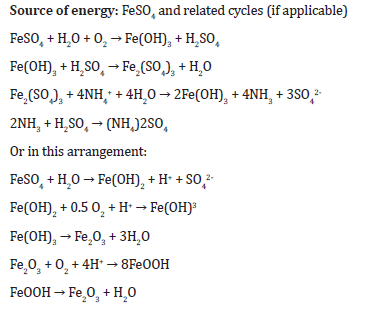
Here is a possible set of biochemical equations without literature of metabolic pathway for the biodesulfurzation of crude oil by Acidithiobacillus ferroxidans:
Also, Pentose Phosphate Pathway from:,br> Ribulose-5-phosphate + 2NADP+ -> Ribose-5-phosphate + CO2 + 2NADPH
As for the amino acid metabolism, Acidithiobacillus ferroxidans is capable of degrading various amino acids to generate energy and nitrogen sources for the cell. One example of an amino acid degradation pathway is the degradation of alanine, which can be represented by the following equation:
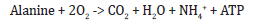
In this reaction, alanine is oxidized to pyruvate, which enters the tricarboxylic acid cycle (TCA cycle) to generate energy. The amino group of alanine is released as ammonia, which can be used as a nitrogen source for the cell.
Nutrient uptake:
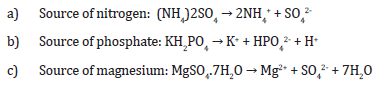
Uptake of sulfur-containing compounds as source of sulfur as the total sulfur from oil (the objective):

The biodesulfurization (BDS) of total sulfur or organosulfur
compounds by Acidithiobacillus ferroxidans involves the following
steps:
A- Uptake of sulfur-containing compounds: Acidithiobacillus
ferroxidans uptakes the sulfur-containing compounds from
the environment, such as dibenzothiophene (DBT) and other
organosulfur compounds from crude oil.
B- Desulfurization: The sulfur-containing compounds are then
desulfurized by the action of enzymes, such as desulfurization
enzymes (Dsz enzymes). These enzymes cleave the carbon-sulfur
(C-S) bonds in the organosulfur compounds, converting them
into smaller sulfur-containing compounds that can be further
metabolized.
C- Metabolism of sulfur-containing compounds: The sulfurcontaining
compounds generated from the desulfurization process
are further metabolized by the sulfur metabolism pathways in
Acidithiobacillus ferroxidans, such as the sulfur oxidation pathway.
In this pathway, sulfur-containing compounds are oxidized to
sulfate, generating ATP and reducing power for the cell.
D- Utilization of sulfate: Sulfate generated from the sulfur
oxidation pathway is utilized as a sulfur source for the biosynthesis
of cellular components, such as proteins and nucleic acids.
Overall, the BDS of total sulfur or organosulfur compounds by Acidithiobacillus ferroxidans involves the uptake, desulfurization, metabolism, and utilization of sulfur-containing compounds.
Biological formations: The reducing equivalents generated during sulfur oxidation and electron transport are used for biosynthetic reactions, including the synthesis of amino acids, nucleotides, and fatty acids. These compounds are needed for the formation of cellular components such as proteins, DNA, and membranes.
A. Enzymes synthesis: enzymes involved in sulfur oxidation (e.g. sulfide: quinone oxidoreductase, sulfur oxygenase reductase)
Sulfide: quinone oxidoreductase:
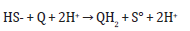
Sulfur oxygenase reductase:
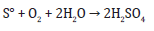
B. Proteins: involved in metabolic processes and cell growth
Protein synthesis: amino acids → proteins
Nucleotides: De novo synthesis of purine nucleotides:
1. PRPP + Glutamine + H2O --> 5-Phosphoribosyl-1-amine +
Glutamate + PPi
2. 5-Phosphoribosyl-1-amine + Glycine + ATP -->
5-Phosphoribosylglycinamide + ADP + Pi
3. 5-Phosphoribosylglycinamide + ATP --> N1-Formyl-5-
phosphoribosylglycinamide + ADP + Pi
4. N1-Formyl-5-phosphoribosylglycinamide + ATP --> N10-
Formyl-5,8,10-trideaza-5,6,7,8-tetrahydrofolate + ADP + Pi
5. N10-Formyl-5,8,10-trideaza-5,6,7,8-tetrahydrofolate
+ Glycine + H2O --> 5,10-Methenyl-5,6,7,8-tetrahydrofolate +
Ammonia + CO2
6. 5,10-Methenyl-5,6,7,8-tetrahydrofolate + H4folate + H+
--> 10-Formyltetrahydrofolate + H2O
7. 10-Formyltetrahydrofolate + Glycine + ATP --> Inosine
monophosphate + ADP + Pi
De novo synthesis of pyrimidine nucleotides:
1. Carbamoyl phosphate + Aspartate -->
N-Carbamoylaspartate + Pi
2. N-Carbamoylaspartate + H2O --> Dihydroorotate + NH3
3. Dihydroorotate + H+ + O --> Orotate + H2O2
4. Orotate + PRPP --> Orotidine-5’-monophosphate + PPi
5. Orotidine-5’-monophosphate + PRPP --> Uridine-5’-
monophosphate + PPi
Note: PRPP stands for 5-Phosphoribosyl-1-pyrophosphate, H4folate stands for tetrahydrofolate, and H2folate stands for dihydrofolate.
C. Electron transport chain: transfer of electrons from Fe2+ to O via quinones, cytochrome
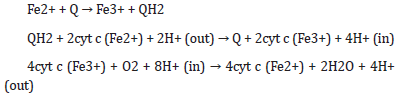
D. Cell divisions: binary fission
Binary fission: one cell → two identical daughter cells.
Other reactions:
A. Fatty acid metabolism: conversion of fatty acids to acetyl-
CoA for energy production
Fatty acid metabolism involves the breakdown of fatty acids to
produce acetyl-CoA, which can then enter the citric acid cycle and
generate energy in the form of ATP. The equation for the conversion
of a generic fatty acid (R-COOH) to acetyl-CoA is:
R-COOH + CoA + ATP → R-CO-S-CoA + ADP + Pi (R-CO-S-CoA is
the activated form of the fatty acid)
The activated fatty acid then undergoes a series of reactions involving beta-oxidation, which results in the formation of multiple molecules of acetyl-CoA. The overall reaction for the complete breakdown of a fatty acid with n carbon atoms is:
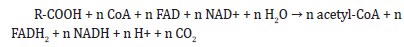
The FADH2 and NADH produced during this process are then oxidized by the electron transport chain to generate ATP.
B. Amino acid metabolism: breakdown of amino acids for
energy and nitrogen metabolism
Amino acid metabolism involves the breakdown of amino acids
for energy and nitrogen metabolism. The process involves the
removal of the amino group through deamination, which results
in the formation of ammonia, which can be used for nitrogen
metabolism. The remaining carbon skeleton can enter different
metabolic pathways depending on the specific amino acid.
An example equation for the breakdown of amino acid alanine
is:
Alanine + NAD+ + H2O → Pyruvate + NH3 + NADH + H+
In this equation, alanine is converted to pyruvate through
deamination, with the release of ammonia. The resulting pyruvate
can then enter the citric acid cycle for further energy production.
C. Pentose phosphate pathway: generates NADPH for
biosynthetic reactions and antioxidant defense
The pentose phosphate pathway involves a series of reactions
that generate NADPH and pentose sugars from glucose-6-
phosphate. The overall reaction can be summarized as:
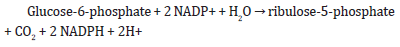
The pathway consists of two distinct phases: the oxidative phase and the non-oxidative phase. In the oxidative phase, glucose-6-phosphate is converted to ribulose-5-phosphate with the concomitant production of two molecules of NADPH. This reaction is catalyzed by glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. In the non-oxidative phase, the pentose sugars produced in the oxidative phase are used in various biosynthetic reactions to generate nucleotides, amino acids, and other important biomolecules. The non-oxidative phase involves a series of reversible reactions that can interconvert between different pentose sugars.
For the given conditions, the pH will increase slightly due to the production of H+ during sulfur oxidation. Electrical conductivity will also increase slightly due to the presence of ions in the media. The API (American Petroleum Institute) will increase due to the removal of sulfur from the crude oil. The growth rate of microorganisms will be low during the first 20 hours and then another low rate for the subsequent 40 hours due to the limited availability of nutrients and the slow growth rate of Acidithiobacillus ferroxidans.
These equations show the oxidation of ferrous iron (Fe^2+) to ferric iron (Fe^3+) with the help of water and oxygen, which is the source of energy for Acidithiobacillus ferroxidans. The ferric iron then reacts with sulfuric acid to form ferric sulfate and water. Ammonium sulfate is the source of nitrogen, while potassium dihydrogen phosphate is the source of phosphate. Magnesium sulfate is the source of magnesium. The microorganism uses the total sulfur from oil to remove sulfur, which is the objective. The microorganism synthesizes enzymes, proteins, and undergoes electron transport chain and cell divisions. Fatty acid metabolism, amino acid metabolism, and the pentose phosphate pathway may also be involved in the metabolic pathway.
To assess the performance of this metabolic pathway, the model must be subjected to a slight increase in pH, a slight increase in electrical conductivity, an increase in API, and low growth rates of microorganisms during the first 20 hours followed by another low rate for the subsequent 40 hours. The operation conditions are 1 atm, 30 °C, and 150rpm.
Optimization
In this work, an optimization for the operation conditions are depending the maximization of growth of microorganisms based on the consumption of salts nutrients. Also, an optimization of the products of pathways are based on the direction of the metabolic pathway.
Partial salts sensing based optimization of microorganisms growth
In this study, optimization based on sensing of partial salts or salinity or salts consumption is presented. The optimization was based on measurement of sensible salts by the applied equipment. It was found that the optimum condisitons are 171rpm, 50 °C, pH 9, surfactant 0.4242 %, OWR 6 for both of microorganisms as shown in Figure 2
Figure 2:Optimum conditions based on sensible salts of both Rhodococcus erythropolis and Acidithiobacillus ferroxidanse.
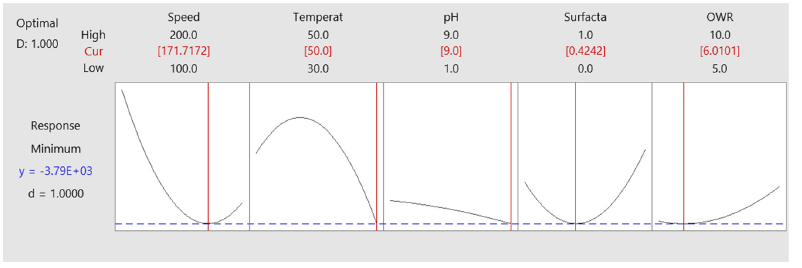
It was found that the optimum conditions are different between the cases of depending the consumption of source of sulfur and nutrienet (28). Therefore, it is recommended in the optimization of biodesulfurization to be multi-varient optimization.
Optimization of pathway products
If the product is elemental sulfur, then the reaction can
be written as follows for Acidithiobacillus ferroxidans and
Rhodococcus erythropolis respectively:
Sulfur-containing compound (e.g. dibenzothiophene) + O2 +
H2O → Elemental sulfur + CO2 + H2O
Sulfur-containing compound (e.g. dibenzothiophene) + 2O2 →
Elemental sulfur + CO2 + H2
In both cases, the sulfur-containing compound is oxidized to elemental sulfur, which is the end product of the reaction.
The choice of product depends on the specific application and desired outcome. Elemental sulfur is a solid product that can be easily removed from the crude oil, while sulfur oxides are gaseous and may require additional processing to remove. However, sulfur oxides have the potential to be converted to sulfuric acid, which can be used in various industrial applications. Additionally, sulfur oxides have a higher value as a sulfur-containing chemical feedstock than elemental sulfur. Ultimately, the decision of which product is better depends on the specific goals of the process and the market demand for the products.
Sulfate (SO42-) is an oxidized form of sulfur that is less harmful to the environment compared to elemental sulfur or sulfur oxides. Sulfate is a common component of natural waters and soils, and it can be easily assimilated by plants and microorganisms for their metabolic processes. Additionally, sulfate can be converted to hydrogen sulfide (H2S) by sulfate-reducing bacteria, which can then be used by sulfur-oxidizing bacteria for further metabolic reactions. Overall, the formation of sulfate as a byproduct of sulfur removal from crude oil is a desirable outcome as it has minimal impact on the environment and can be further utilized by other organisms in the ecosystem.
Conclusion
Biodesulfurization is a promising technology for reducing the sulfur content of fossil fuels, which can help to reduce harmful emissions during combustion. Rhodococcus erythropolis and Acidithiobacillus ferroxidans are effective and efficient bacteria in BDS of oil. The performance of biodesulfurization can be evaluated by monitoring various physicochemical parameters, such as pH, temperature, and the concentration of organic and inorganic compounds. By carefully monitoring these parameters, it is possible to optimize the conditions for BDS and achieve the highest possible desulfurization efficiency. A suggested model for BDS can take into account the various physicochemical changes that occur during the process and adjust the parameters accordingly to ensure optimal performance. Additionally, statistical optimization techniques can be used to determine the optimal conditions for BDS, such as the maximization of consumption of salts medium, which can help to further improve the efficiency and effectiveness of the process. By combining physicochemical monitoring with statistical optimization, it is possible to develop a robust and effective biodesulfurization process for reducing sulfur content in fossil fuels.
References
- Sadare OO, Obazu F, Daramola MO (2017) Biodesulfurization of petroleum distillates - current status, opportunities and future challenges. Environments 4(4): 85.
- Mujahid A, Maryam A, Afzal A, Bajwa SZ, Hussain T, et al. (2020) Molecularly imprinted poly (methyl methacrylate)-nickel sulfide hybrid membranes for adsorptive desulfurization of dibenzothiophene. Separation and Purification Technology 237: 116453.
- Chen S, Zhao C, Liu Q, Zhang X, Sun S, et al. (2019) Biodesulfurization of diesel oil in oil–water two phase reaction system by Gordonia sp. SC-10. Biotechnology letters 41: 547-554.
- Abro R, Abdeltawab AA, Al-Deyab SS, Yu G, Qazi AB, et al. (2014) A review of extractive desulfurization of fuel oils using ionic liquids. Rsc Advances 4(67): 35302-35317.
- Siddiqui SU, Ahmed K (2016) Methods for desulfurization of crude oil-a review. Science International 28(2): 1169-1173.
- Duissenov aPaposcang-cdtisrNUoSaT.
- Hamad EZ A-SE, Al-Qahtani AS (2013) Desulfurization of whole crude oil by solvent extraction and hydrotreating. Saudi Arabian Oil Co.
- Pacheco M, Paixao SM, Silva TP, Alves L (2019) On the road to cost-effective fossil fuel desulfurization by Gordonia alkanivorans strain 1B. RSC advances 9(44): 25405-25413.
- Gunam IB YK, Sujaya IN, Antara NS, Aryanta WR, Tanaka M, et al. (2013) Biodesulfurization of dibenzothiophene and its derivatives using resting and immobilized cells of Sphingomonas subarctica T7b. J Microbiol Biotechnol 23(4): 473-482.
- Kirkwood KMJMFÆMRGSaoscio-at-l-pcoRssJ.
- Speight JG, El-Gendy NS (2017) Introduction to petroleum biotechnology. Gulf Professional Publishing.
- Nassar HN, Ali HR, El-Gendy NS (2021) Waste prosperity: Mandarin (Citrus reticulata) peels inspired SPION for enhancing diesel oil biodesulfurization efficiency by Rhodococcus erythropolis HN2. Fuel 294: 120534.
- Li FL, Xu P, Ma CQ, Luo LL, Wang XS (2003) Deep desulfurization of hydrodesulfurization-treated diesel oil by a facultative thermophilic bacterium Mycobacterium sp. X7B. FEMS Microbiology Letters 223(2): 301-307.
- Li WXJEobobbasWJMBSSBMBV.
- Alves L, Paixão SM, Pacheco R, Ferreira AF, Silva CM (2015) Biodesulphurization of fossil fuels: energy, emissions and cost analysis. RSC Advances 5(43): 34047-3457.
- Boshagh F, Mokhtarani B, Mortaheb HR (2014) Effect of electrokinetics on biodesulfurization of the model oil by Rhodococcus erythropolis PTCC1767 and Bacillus subtilis DSMZ 3256. Journal of Hazardous Materials 280: 781-787.
- Ismail W E-SW, Abdul Raheem AS, Mohamed ME, El Nayal AM (2016) Biocatalytic desulfurization capabilities of a mixed culture during non-destructive utilization of recalcitrant organosulfur compounds. Frontiers in Microbiology 3(7): 266.
- Karimi E JC, Yazdian F, Akhavan SA, Hatamian A, Rasekh B, et al. (2017) DBT desulfurization by decorating Rhodococcus erythropolis IGTS8 using magnetic Fe3O4 nanoparticles in a bioreactor. Engineering in Life Sciences 17(5): 528-535.
- Martin AB, Alcon A, Santos VE, Garcia-Ochoa F (2005) Production of a biocatalyst of Pseudomonas p utida CECT5279 for DBT Biodesulfurization: Influence of the operational conditions. Energy & Fuels 19(3): 775-782.
- Martínez I, Mohamed ME-S, Rozas D, García JL, Díaz E (2016) Engineering synthetic bacterial consortia for enhanced desulfurization and revalorization of oil sulfur compounds. Metabolic Engineering 35: 46-54.
- Martinez I, Santos VE, Alcon A, Garcia-Ochoa F (2015) Enhancement of the biodesulfurization capacity of Pseudomonas putida CECT5279 by co-substrate addition. Process Biochemistry 50(1): 119-124.
- Dejaloud A VF, Habibi A (2017) Ralstonia eutropha as a biocatalyst for desulfurization of dibenzothiophene. Bioprocess and Biosystems Engineering 40(7): 969-980.
- Awadh M, Mahmoud H, Abed RM, El Nayal AM, Abotalib N, et al. (2020) Diesel-born organosulfur compounds stimulate community re-structuring in a diesel-biodesulfurizing consortium. Biotechnology Reports 28: e00572.
- Yi Z, Ma X, Song J, Yang X, Tang Q (2019) Investigations in enhancement biodesulfurization of model compounds by ultrasound pre-oxidation. Ultrasonics Sonochemistry 54: 110-120.
- Feng SXL, Yanjun T, Xing H, Hailin Y. Biodesulfurization of sulfide wastewater for elemental sulfur recovery by isolated halothiobacillus neapolitanus in an internal airlift loop reactor. Bioresource Technology 264: 244-252.
- Ahmed ME, Zafar AM, Hamouda MA, Aly Hassan A, Arimbrathodi S (2022) Biodesalination research trends: A bibliometric analysis and recent developments. Sustainability 15(1): 16.
- Davardoostmanesh M, Ahmadzadeh H (2023) Remediation of saline oily water using an algae-based membrane. Journal of Membrane Science 666: 121201.
- Al-Khazaali W, Ataei S (2023) Optimization and utilization of biodesulfurization of heavy sour crude oil.
© 2023 Seyed Ahmad Ataei. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






