- Submissions

Full Text
Research & Development in Material Science
Synergistic Effect of 30 B and 15 A Organoclays and Compatibilizer on Improved Barrier Properties of HDPE/PA6 Composites: Preparation and Characterization
Azita Alipour* and Naser Sharifi Sanjani
Polymer Laboratory, Chemistry Department, School of Science, University of Tehran, Iran
*Corresponding author:Azita Alipour, Polymer Laboratory, Chemistry Department, School of Science, University of Tehran, Tehran, Iran
Submission: August 28, 2023;Published: September 26, 2023

ISSN: 2576-8840 Volume 19 Issue 3
Abstract
Rigid containers constructed from high density polyethylene (HDPE) have high permeability toward hydrocarbons and organic molecules. This problem causes loss of fuel from gasoline tank of automobiles. Polyamide 6, organoclays (Oclays) and compatibilizer (Cp) are effective components for increasing of barrier properties of polyethylene. Three-component nanocomposites composed of HDPE/HDPE-g-MAH/PA6/Oclay were produced via twin-screw co-rotating extruder melt mixing method. X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) analyses showed the exfoliation of nanoparticles in the nanocomposites. Oclays and Cp increased melt viscosity and also modulus, and tensile strength (TS). Oclays and Cps caused a heightening effect on the barrier properties of samples. Among Oclays, the 15A showed better performance in properties compared to 30B Oclay due to the lower polarity of 15A compared to 30 B and its greater compatibility with the polymer matrix.
Keywords: High density polyethylene; Polyamide 6; Maleic anhydride grafted polyethylene (HDPE-g-MAH); Blend; Nanocomposite; Barrier properties
Graphical abstract
Figure 1:
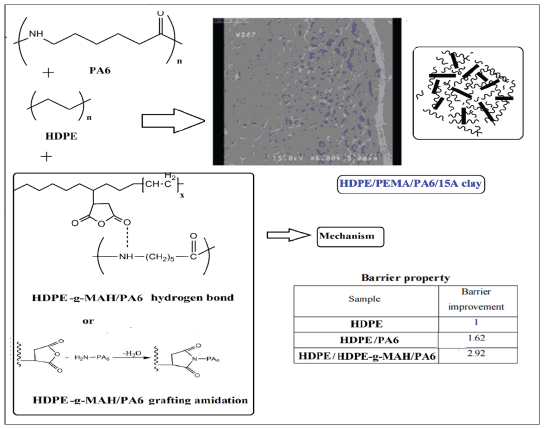
Introduction
In the last several decades, blending two or more polymers has been known as a practicable and common procedure to produce materials with new and desirable properties, different from those of the constituents [1-3]. However, in the immiscible blends, due to phase incompatibility, it is usually difficult to obtain products with a tailored final performance. For solving this problem, it is necessary to incorporate a Cp into the system to enhance phase domain interfacial interaction and subsequently the final performance of the product [4-6]. One type of Cp that can be used for this purpose is reactive Cp containing suitable functional groups to create chemical interactions between the system constituents in the molten state to form a fine-interfacial polymer composite [7-9]. Rigid containers made of HDPE are widely used for various household and industrial packaging. This polymer has weak resistance against organic solvents which reduces its ability for some special applications that require high resistance toward organic hydrocarbons such as cars’ gasoline tanks [8,10,11]. In contrast, PA6 due to the existence of polar sites and intermolecular hydrogen bonding has good diffusion resistance toward organic solvents. Thus, blending PE and PA6 should be an efficient way to fabricate a product with good final properties, because the shortcomings of each of the polymers are compensated by the other polymer [12-14]. However since HDPE/PA6 blends are thermodynamically immiscible, Cp is often needed to modify the interfacial properties and final performance of the product [4-6]. In addition, it was reported that the processing conditions, the presence of nanofillers, and the state and extent of dispersion of nanofillers can influence on the improvement of physico-chemical and impermeability properties [13-15]. The main objective of this work is the improvement of the barrier property of polyethylene toward hydrocarbon solvents by adding PA6, HDPEg- MAH as Cp and Oclays as nanofiller. The samples were prepared via melt compounding in the presence of two types of Oclays (30B and 15A), and studied in terms of the morphological, rheological, mechanical, thermal and barrier properties. The influence of the employed Cp and Oclays was evaluated on the improvement of the properties and the efficiency of the nanocomposites in the present work. The novelty of this work is the construction of a three components polymer composite composed of HDPE/PA6/HDPE-g- MAH/Oclay and the effect of these components on the improvement of physicomechanical and barrier properties of HDPE with the aim of development in the plastic and automotive industry. Here we investigate the effect of the type of nano clays and their synergy effect and also the presence of Cp on physical-mechanical and barrier properties of composites and comparison between them. We also examined the mechanism of the performance of Cp in the composite. And we showed that the presence of Cp and OClay led to a significant improvement in the properties of nanocomposites.
Experimental
Materials
Polymeric constituents used in this work were HDPE (5218 grade, MFI (190 ˚C/2.16kg) =18.0, m.p=131 ˚C, density=952kg m-3, Tabriz Petrochemical, Iran) as the continuous phase and PA6 (textile grade, Dutch State Mines Company (DSM), Netherlands) as dispersed phase. HDPE-g-MAH with maleic anhydride content of 1.5% was utilized as a Cp. The nanofillers used in this work were Cloisite 30B (a natural montmorillonite modified with a quaternary ammonium methyl bi-2-hydroxyethyl tallow salt) and Cloisite 15A (a natural montmorillonite modified with a quaternary ammonium of dimethyl dehydrogenated tallow salt).
Processing and sample preparation
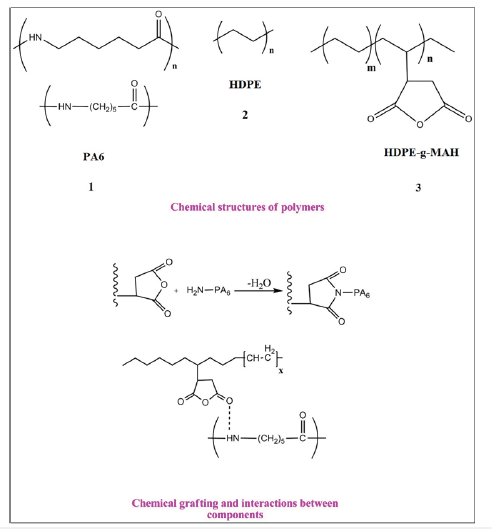
The samples were prepared via melt mixing in a co-rotating twin-screw extruder (screw length (L)= 80cm, screw diameter (D)=2cm, L/D=40cm, Brabender, Germany). Before mixing, PA6 and Oclays were dried at 80 °C for 24h under vacuum conditions to prevent the influence of moisture and hydrolytic degradation on the samples. HDPE and HDPE-g-MAH were also dried in an oven at 60 °C for 12h. The materials were first premixed in the solid state and then fed into the extruder. The composites were prepared by reactive extrusion coupled with hot stretching. The extruder operated with a thermal profile of 180 ºC, 200 ºC, 228 ºC, 230 ºC, 235 ºC and 245 °C at a screw speed of 80rpm and residence time of 120s. The molten material exiting from the die was cooled in a water bath, dried by air and then cut into granular particles. The formulations and compositions of the samples are reported in Table 1. The schematic representation of the chemical structure of the components and chemical interaction and grafting between components are shown in Figure 1; [16,17].
Table 1:Formulation and composition of the samples prepared in the present work.
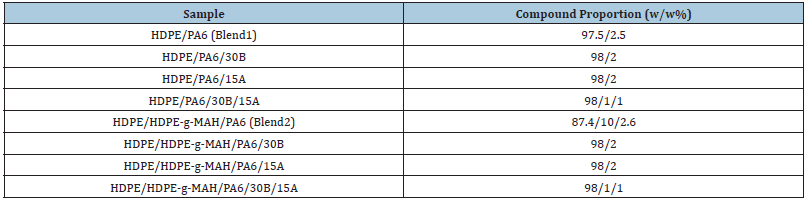
Figure 2:Schematic representation of the chemical structure of the components and chemical interaction and grafting between components.
Characterization
Scanning Electron Microscopy (SEM): The morphology of the samples was characterized using a FESEM Hitachi S-4160 apparatus (Japan) on the samples fractured in liquid nitrogen. Before observation, the fracture surfaces were coated with a thin layer of gold to make them conductive.
X-ray Diffraction (XRD): XRD analyses were performed to specify the state and degree of dispersion of Oclays and the nano-scale morphology formed in the nanocomposites. The diffractograms were acquired in the diffraction angles between 2 and 10º. The analyses were carried out using an Xpert MPD x-ray diffractometer (Philips, Netherlands) with Co Kα radiation and wavelength of 1.78898 A˚ operating at 40kV voltage and 30mA current, at room temperature.
Rheological measurements: The rheological properties and Melt Flow Index (MFI) values of the samples were investigated via a Quick index CEAST ITALY MFI apparatus at 190 °C with a load of 2.16kg according to the ASTM D1238 standard.
Mechanical measurements: Melt-blended granular specimens obtained from the extruder were utilized to produce dumbbellshaped specimens for the mechanical tests. The dumbbell-shaped specimens were produced via injection molding using an injection molding machine (Aslanian, Iran, with a reservoir capacity of 120g). The temperatures in the three zones of the barrel were 225 ºC, 235 ºC and 245 °C and injection was carried at mold temperature of 250 °C, loading speed of 80rpm, injection pressure of 80 bar and cooling time of 70s. The tensile tests were performed in accordance with ASTM D638 standards using a GOTECH Al-3000 tensile testing machine (GOTECH, Korea) with a crosshead speed of 50mm/min on the dumbbell-shaped injection molded samples. Approximately three to five dumbbells were used for each sample to obtain the mechanical properties.
Thermogravimetric Analyses (TGA): To examine the thermal stability of the samples, TGA analyses were conducted from room temperature to 600 °C with a heating speed of 10C/min by using a TGA Q50 V6.3 Build 189 (TA Instrument, Germany) thermal source. Before the analyses, all of the specimens were dried in an oven at 60 °C for 24h under vacuum conditions to prevent the influence of the absorbed moisture and the resulting hydrolytic degradation on the samples.
Differential Scanning Calorimetry (DSC): The melting and crystallization behavior of the samples was inspected via DSC analyses by using a DSC Q 100 V9.0 Build 275 (TA Instrument, Germany) apparatus at a heating rate of 10 °C/min from -80 °C to 250 °C. Before the experiments, all of the specimens were dried at 80 °C in an oven under vacuum conditions to prevent the effect of moisture on the samples.
Barrier measurements: The barrier measurements were conducted on hot-pressed sheets in 200μm thickness which was produced at a hot-pressed temperature of 250 °C and a cooling time of 9min in a Mini test press apparatus (Toyoseiki, Japan). The sheets were cut into circular plates, then sealed as lids on the top of flasks filled with xylene. The barrier properties of samples were determined by measuring the weight loss of the xylene after putting the flasks at 40 °C for 7 days. The results were reported in the form of barrier improvement parameter (g/day) by measuring the weight loss of the samples after contact with xylene.
Results and Discussion
Morphological observations
Figure 3:SEM images of A) HDPE/PA6, A1) HDPE/PA6/30B, A2) HDPE/PA6/15A, A3) HDPE/PA6/30B/15A, B) HDPE/HDPE-g-MAH/PA6, B1) HDPE/ HDPE-g-MAH /PA6/30B, B2) HDPE/HDPE-g-MAH/PA6/15A and B3) HDPE/ HDPE-g-MAH /PA6/30B/15A.
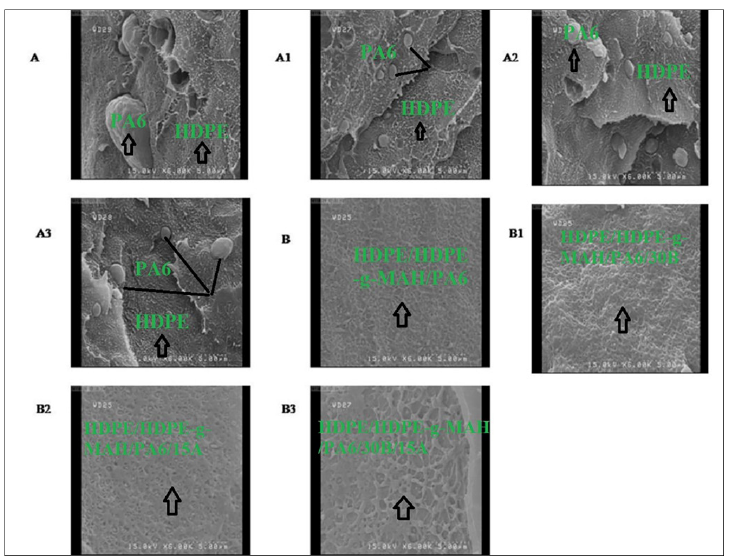
To characterize the surface morphology, the dispersion state of the polymer phases and reciprocal adhesion between the phases, SEM images obtained from the fracture surfaces of the samples are shown in Figure 2. The SEM images of the HDPE/PA6 samples (2A-A3) showed a heterogeneous morphology with typical seaisland structure wherein the minor phase (PA6) was dispersed in the shape of spherical domains in the continuous HDPE matrix; distinct and clear interfaces can be observed in the micrographs. This morphology can be interpreted due to the weak affinity and high interfacial tension between the two polymeric phases caused by the different polarity and chemical structure of the components. In the SEM images of the samples with Cp (2B-B3) a more uniform morphology was observed and the dispersed PA6 domains were no longer visible in the presence of the Cp. In these samples due to finely and homogeneously dispersion of the polymer phases, there is a rough and indistinct interface; in other words, a clear and separate phase domain couldn’t be seen in these blends. The Cp molecules via increasing the interfacial adhesion and compatibility between the polymeric phases, improved the phase morphology in the blend. The common chemical mechanism recognized for this compatibility is that a grafting chemical reaction occurred between the maleic anhydride groups of the HDPE-g-MAH molecules and the amide and terminal amino groups of PA6 at the PE-PA interface to in-situ form a PA-g-Cp copolymer. The formed copolymer then resulted in the covalent intermolecular interactions between PE and PA and the connection and compatibility between polymeric phases [18-20]. According to the SEM images of the HDPE/PA6 nanocomposites (Figure 2A1-A3), the presence of the Oclays caused a notable reduction in the size of the PA6 spherical particles and the improvement of the phase dispersion. The Oclays, by reducing the interfacial tension and energy between the immiscible phases, increase the interaction force between phases and as a result promote dispersion of the PA6 phase [21-23]. The Oclays, via increasing the melt viscosity, cause the collapse of the dispersed phase particles and consequently a more homogeneous dispersion of the phases. Moreover, the inserted Oclays in the interface of the polymeric phases increase affinity between the phases and reduce the aggregation of the dispersed phase particles in the blend and consequently cause the improvement of the phase morphology.
X-ray analyses
The XRD analyses were performed on the nanocomposites to examine the state and degree of delamination of the Oclays. The diffraction patterns were recorded from 2θ of 2-12º and the diffractograms are shown in Figure 3 & 4. The XRD patterns of 30B indicated the characteristic peaks at 2Ɵ=6.5º and 15A showed two visible peaks at 2Ɵ= 4º and 8.4º corresponding to the (001) plane reflection of clays with dspacing (001) (Aº). The middle line (b), associated with pure Oclay 15 silica, is a constant line since this is an amorphous substance. The X-ray pattern of the nanocomposites indicated the disappearance of the characteristic peak of the Oclays which confirm the complete separation and exfoliation morphology of Oclays in the composite matrix [4,5,20]. The hydrogen bonding between the amino groups of PA6 and the hydroxide groups on the surface of the clay layers promotes the entrance of the polymer molecules into the interlayer space of the nanoparticles, and as a result, facilitates the dispersion of the layers. This indicates the role of PA6 in the delamination of the clay layers. In addition, according to the role of the HDPE-g-MAH as Cp in the increase of the compatibility between the polymeric phases as well as its effect on increasing of the melt viscosity, it can be inferred that Cp molecules had an accelerating effect on the separation and dispersion of the clay layers in the blend. The Cp molecules increasing the melt viscosity led to the increase of the shear tensions in the molten state and as a result the failure of the clay tactoids also increasing of the compatibility between the polymer phases and the Oclays promoted the dispersion of the nano clays and the translocation of the macromolecules into the interlayer space of the clay layers [20-22].
Figure 4:X-ray patterns of a) Clay30B, b) Clay15A, c) HDPE/PA6/30B, d) HDPE/PA6/15A and HDPE/ PA6/30B/15A.
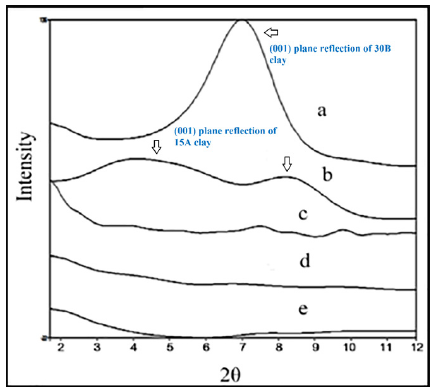
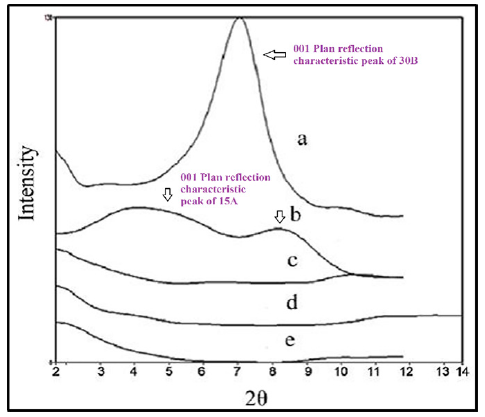
Figure 5:X-ray patterns of a) Clay30B, b) Clay 15A, c) HDPE/HDPE-g-MAH/PA6/30B, d) HDPE/HDPE-g-MAH/ PA6/15A and e) HDPE/HDPE-g-MAH/PA6/30B/15A.
Rheological properties
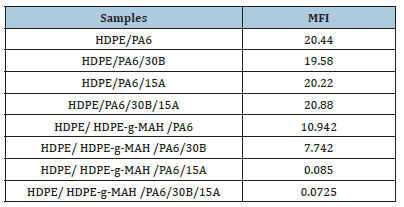
To evaluate the rheological properties of the samples, MFI analyses were conducted on all the materials and the results are presented in Table 2. According to the results, the samples containing Cp had lower melt indices than the samples without Cp. Adding the Cp to the HDPE/PA6 blend reduced the melt index by about 1.86 times. The chemical grafting interactions between the anhydride groups of the Cp and the amino groups of PA6 would be expected to increase the MW of the polyamide molecules and the molecular entanglements and consequently the melt viscosity of the blend. Also, the Cp molecules by forming the compatibility between the immiscible phases of HDPE and PA6 restrict the sliding of the melt units upon each other as a result, increase the melt viscosity. These observations are in close agreement with the results reported by other studies [21-23] The nanoparticles had a considerable increase in the melt viscosity of the HDPE/ HDPEg- MAH/PA6 samples. The strong interactions between the clay layers and the polymer molecules confined between the layers restricted the movement of the molecules and in consequence, led to increasing the melt viscosity. The greater effect of the 15A nanoparticles on the melt viscosity relative to the 30B nanoparticles can be explained by their compatibility with the HDPE matrix. The MFI results, however, did not show any perceptible change for the HDPE/PA6 samples in the presence of the Oclays which can mostly be due to the incompatibility of the phases in the blend. Although although the presence of the Cp and Oclays was effective in the increase of the melt viscosity of the HDPE/ HDPE-g-MAH/PA6 samples, it seems that the existence of the Cp alone had a significant effect on the enhancement of the melt viscosity due to the increase in the interfacial adhesion between the phases.
Table 2:Melt index values of the blends and related nanocomposites.
Mechanical properties
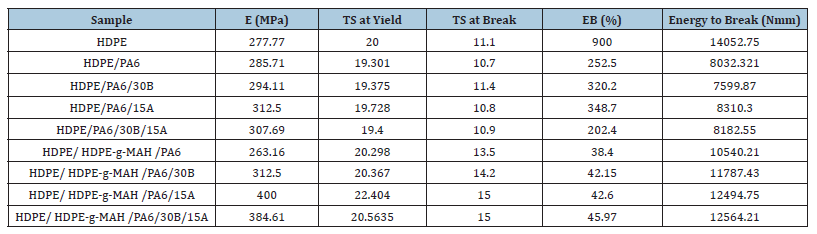
The mechanical properties, including elastic modulus (E), Tensile Strength (TS), Elongation at Break (EB) and energy to break, are reported in Table 3. The HDPE/PA6 blend displayed higher modulus than the pure polyethylene and the blends containing Cp (HDPE/PA6/ HDPE-g-MAH) showed higher modulus than the blends without Cp which indicated increasing rigidity of the system in the presence of HDPE-g-MAH. On the other hand, the presence of Oclays as expected caused the increase of modulus of the HDPE/PA6 blend. But the results showed that the addition of PA6 led to lower values in elongation and energy to break for the HDPE/PA6 blend relative to the pure polyethylene. The weak interfacial adhesion and poor compatibility between the phases which lead to reducing the deformability and forming defect points between the phases can be a reasonable explanation for the above result. The neat HDPE showed more elongation and energy to beak than the blends, and in addition, the incorporation of Cp caused a significant reduction in elongation relative to the HDPE/ PA6 blends. The presence of Cp due to the formation of interchain interactions and hydrogen bonds causes the increase of stiffness of the blend and as a consequence reduction of elongation. The lower strength of HDPE-g-MAH molecules relative to the HDPE molecules as a result of the existence of the maleic anhydride groups can be another reasonable explanation for this result. This result has been also shown in other studies [5,17]. The higher tensile strength and energy to break were also observed for the samples with Cp relative to the samples without Cp. By adding the Cp to the HDPE/ PA6 blend because of increasing the compatibility between the phases the tensile strength of the HDPE is increased but elongation is considerably reduced, which can be due to the increase of rigidity of the system. In other words, the Cp molecules by increasing the interfacial adhesion and reducing the defect points between the phases arising from the chemical grafting interactions between HDPE-g-MAH and PA6 led to increasing of energy to break. Results reported by other groups support the above observations [24-26]. The presence of the Oclays increased the modulus and energy to break values of the HDPE/PA6 and HDPE/ HDPE-g-MAH /PA6 blends but had a negligible effect on the elongation and tensile strength values of the nanocomposites. The Oclays by creating immobile or slightly mobile polymeric phases arising from the strong interactions between with the polymer molecules reinforce the mechanical performance of the product. The influence of nanoparticles on the improvement of mechanical properties was also observed in prior works [27-29].
Table 3:Mechanical properties of HDPE, the blends and the nanocomposites.
Thermal properties
Differential scanning calorimetry: The melting and crystallinity behavior of the samples was characterized through DSC analyses. The DSC parameters (melting temperature (Tm), melting enthalpy (ΔHm) and also Tg) are reported in Table 4. DSC curves of the samples are shown in Figure 5 & 6. The DSC thermograms of the samples showed a sharp peak at 129 °C which is attributed to the crystalline and melting temperature of composite which due to more HDPE content it is closer to the melting point of polyethylene. The addition of the 15A nanoparticles and the 30B and 15A mixture to the blends of HDPE/PA6 and HDPE/ HDPE-g- MAH/PA6 increased the Tm of the HDPE somewhat while the 30B nanoparticles reduced slightly the Tm of the HDPE. The different behavior of the 30B nanoparticles can be interpreted regarding their high polarity and low compatibility with the matrix. It is surmised that the 15A nanoparticles because of their lower polarity and more compatibility with the HDPE molecules diffused easily into the nonpolar space of the matrix and by forming the strong interactions with the polymer molecules restricted the mobility of the molecules that led to the increased of Tm. The Oclays with increasing the structural order promote a regular orientation and arrangement of the macromolecules in the blends and in this way facilitate the crystallization of the molecules and in consequence, increase the melt temperature. The melting temperature of HDPE in the blend with Cp was lower than that of the blend without Cp. Because of the chemical interactions between the anhydride groups of HDPE-g-MAH and the terminal amino groups of PA6, the coupling structures would be produced between HDPE and PA6 which would restrict the movement and folding of the polymer links into the crystalline regions. Therefore, the crystallization would become much more difficult and the perfection and crystallizability of polymer molecules would be decreased. This reduction leads to the reduction of crystallinity of the blend with Cp relative to the blend without Cp. This reduction can be also attributed to the lower crystallinity of the Cp relative to other components. The results reported by previous studies support the above observations [7,10]. The Tg of HDPE is -110 ºC and Tg of PA6 is 45-50 ºC and composition of these two compounds in fact has a combined temperature of these two points in about 12. The glass transition temperature (Tg) is the temperature at which the polymer changes from the glassy state to the rubbery state. As can be observed the Tg point of the nanocomposites were appeared at the stair point in the curve at a temperature of about 12 degrees. The Tg point values of HDPE increased by adding the nanoparticles to the blends. Since Tg is related to the long-range movements of molecules, it can be conjectured that the strong interactions between the clay layers and the polymer chains caused a restriction in the movement of the molecules and therefore caused the increase in Tg [5,7,10]. The melting point of nanocomposites has appeared at a temperature of 130 degrees, as an endothermic peak which is closer to the melting temperature of polyethylene. The melting temperature of polyethylene is 130 degrees and the melting temperature of polyamide is 250 degrees. The melting temperature of the composite is a combination of the melting point of two components and closer to the melting temperature of polyethylene due to the main phase of polyethylene forming the system.
Table 4:DSC parameters of the prepared samples.
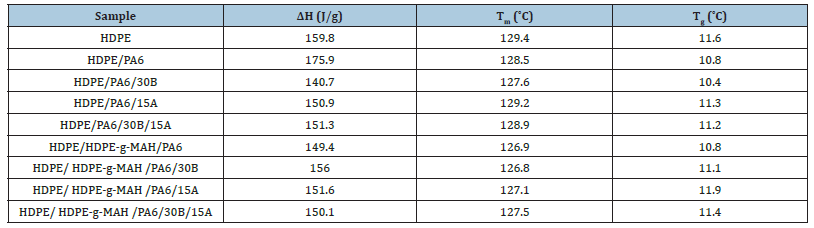
Figure 6:DSC curves of a) HDPE/PA6, b) HDPE/PA6/30B, c) HDPE/PA6/15A and d) HDPE/PA6/30B/15A.
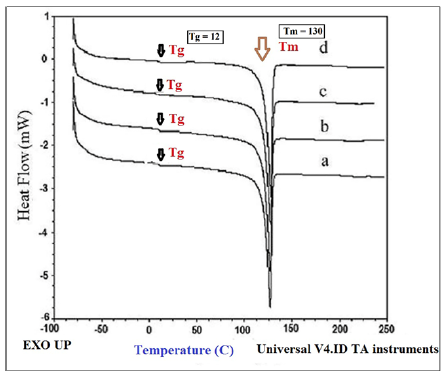
Figure 7:DSC curves of a) HDPE/HDPE-g-MAH/PA6, b) HDPE/HDPE-g-MAH/PA6/30B, c) HDPE/HDPE-g-MAH/ PA6/15A and d) HDPE/HDPE-g-MAH/PA6/30B/15A.
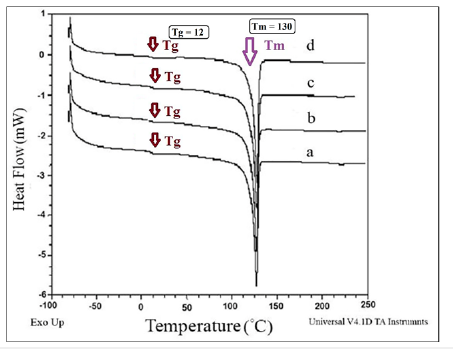
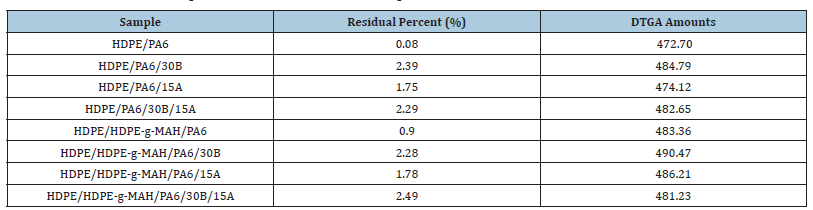
Thermogravimetric analyses: To inspect the thermal stability and degradation resistance, TGA analyses were performed on the samples; the curves are shown in Figure 7 & 8. The TGA results including residual weight values (%) and also DTGA amounts are given in Table 5. According to the results reported in Table 5, it can be inferred that the Oclays caused the increase in the thermal stability of the nanocomposites relative to the pure blends. The degradation of the nanocomposites began at higher temperatures than the pure blends without nano clay. According to the DTGA results, the 50% degradation temperature of the nanocomposites was shifted to higher temperatures in comparison with the pure blends indicating the effect of the Oclays on the increase of the thermal resistance. The insulator carbon layer formed on the surface of the nano clays increased the resistance of the matrix against the thermal degradation and obstructed the volatile decomposition products inside the nanocomposites from diffusing out. The strong interactions between the clay layers and the polymer molecules restrict the thermal mobility of the molecules and as a result, increase the resistance of the polymer matrix against thermal changes. These observations supported the nanoscale morphology formed by the Oclays in the nanocomposites. The results showed lower thermal stability for the pure blend with Cp relative to the pure blend without Cp which confirms the DSC results and lower crystallizability of HDPE/HDPE-g-MAH/PA6 relative to HDPE/PA6 because the low crystallinity leads to the decrease of the thermal stability.
Figure 8:TGA curves of a) HDPE/PA6, b) HDPE/PA6/30B, c) HDPE/PA6/15A and d) HDPE/PA6/30B/15A.
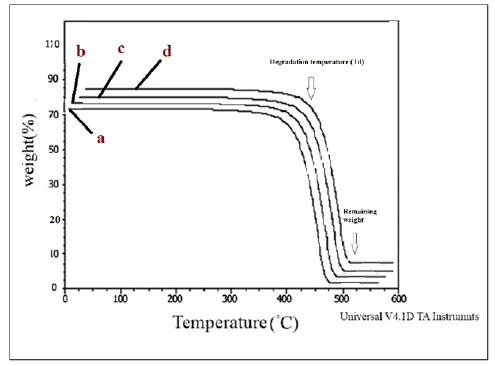
Figure 9:TGA curves of a) HDPE/HDPE-g-MAH/PA6, b) HDPE/ HDPE-g-MAH /PA6/30B, c) HDPE/ HDPE-g-MAH / PA6/15A, d) HDPE/ HDPE-g-MAH /PA6/30B/15A.
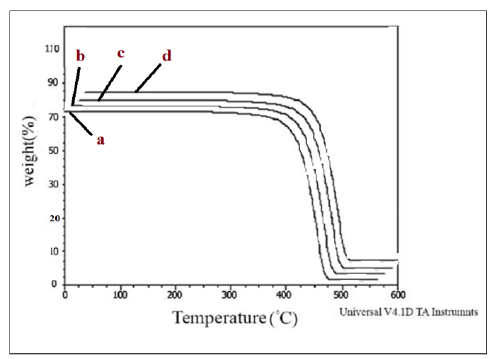
Table 5:TGA traces of the produced blends and nanocomposites.
Barrier properties
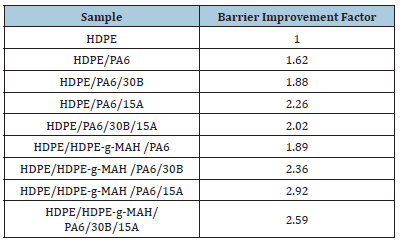
The barrier properties of the samples, in terms of barrier improvement parameter, are reported in Table 6. All samples showed better barrier properties than the pure polyethylene did and the samples with Cp displayed better barrier properties relative to the samples without Cp. This can be attributed to the presence of the Cp in the blend and consequently the reduction of the free volume between the immiscible phases caused by the chemical grafting interactions between the Cp and polyamide molecules and subsequently the increase of the interfacial adhesion of the phases. These interactions limited the diffusion of the nonpolar xylene molecules and thereby enhanced the permeation resistance against the diffusion of organic molecules. The presence of polyamide also improved the barrier property of the blends relative to the pure polyethylene. The polar amide and amino groups of polyamide as well as intermolecular hydrogen bond, via forming polar sites, restricted the diffusion of the nonpolar xylene molecules which can easily penetrate through the nonpolar amorphous regions of polyethylene. The nonpolar xylene molecules can hardly diffuse through the crystalline areas of polymers and prefer to percolate mostly through the amorphous regions. Therefore, it can be suggested that the molecular composition and the configuration of the amorphous areas can greatly influence barrier properties [29-31]. Since the temperature of the analyses (40 ˚C) was higher than Tg of polyethylene and lower than Tg of polyamide, it can be inferred that the amorphous segments of polyethylene were more flexible than those of polyamide. These flexible and nonpolar regions can provide an easier diffusion path for the molecules than the rigid, dense and polar amorphous regions of polyamide. The addition of nano clays into the blends improved the barrier properties. The nanofillers reinforce the permeation resistance of the blends by producing tortuous diffusion paths. The permeation of the molecules was limited by the HDPE matrix composed of impermeable Oclays and polyamide particles because of the tortuous diffusion path created by these particles. Therefore, the barrier results verified the synergetic effect of compatibility and nanoparticles on the reinforcement of barrier properties. According to the results, the 15A nanoparticles indicated a greater effect on the improvement of the barrier properties of the samples compared with the 30B nanoparticles. This can be explained regarding to the lower polarity and more compatibility of these particles with the polyethylene matrix [32,33].
Table 6:The barrier improvement tracers of the samples studied in the present work.
Conclusion
In this work, blends and nanocomposites composed of HDPE and PA6 with and without HDPE-g-MAH as Cp with Oclays of 30B and 15A as nanofiller were prepared by the method of melt compounding. The results showed that the Cp and Oclays had a substantial effect on the improvement of the morphology and properties of the samples. The SEM analyses indicated a homogeneous morphology in the presence of the Cp. The XRD patterns showed the separation of the clay layers and the exfoliated morphology in the nanocomposites. A noticeable increase was observed in the melt viscosity in the presence of the Cp and Oclays. Regarding the mechanical results, the samples with Cp indicated higher tensile strength and energy at break than the samples without Cp showing the increase of the interfacial interactions and the reduction of the defect regions between the immiscible phases by adding the Cp. The DSC results showed the reduction of the crystallizability in the presence of the Cp and the increase of crystallizability in the presence of the nanofillers. The increase in thermal stability in the presence of the Oclays was also confirmed by the TGA results. The samples containing Cp (HDPE/HDPE-g-MAH/ PA6) revealed higher barrier properties than the samples without Cp (HDPE/PA6) and the Oclays, especially 15A nanoparticles, displayed a significant influence on the reinforcement of the barrier properties. Summing up the observations resulting from the morphological, thermal, rheological, mechanical and barrier properties showed that the presence of the Cp and nanoparticles especially the 15A nanoparticles had a more effect on the improvement of the properties.
Acknowledgement
The progress of this project owes a lot to the assistance of Mr. Abas Fatehi and Ali Ranji at the Iran Polymer and Petrochemical Institute. The effective support of Mr. Hamid Mohammad Hosseini and Mr. Hasan Hasani at the Plastic Process Workshop at Iran Polymer and Petrochemical Institute in the production of the samples is also admirable. In addition, the authors appreciate Mr. Davood Khademi in Arianam Engineering Company for his help in the determination of the mechanical features.
Competing Interests and Funding
The authors don’t have any conflict of interest for this journal and this work is not supported by any funding or financial support.
References
- Ferri JM, Garcia-Garcia D, Rayon E, Samper MD, Balart R (2020) Compatibilization and characterization of polylactide and biopolyethylene binary blends by non-reactive and reactive compatibilization approaches. Polymers 12(6): 1344.
- Zhao X, Wang H, Fu Z, Li Y (2018) Enhanced interfacial adhesion by reactive carbon nanotube: new route to high-performance immiscible polymer blend nanocomposites with simultaneously enhanced toughness, tensile strength, and electrical conductivity. ACS Appl Mater Interfaces 10: 8411-8416.
- Huang Y, Kormakov S, He X, Gao X, Zheng X, et al. (2019) Conductive polymer composites from renewable resources: an overview of preparation, properties, and applications. Polymers 11(2): 187.
- Erdmann E, Dias ML, Pita V, Destefanis H, Monasterio F, et al. (2007) Characterization of HDPE /polyamide 6/nanocomposites using scanning and transmission electron microscopy. Macromol Symp 258: 82-89.
- Scaffaro R, Botta L, Mistretta MC, La Mantia FP (2010) Preparation and characterization of polyamide 6/polyethylene blend-clay nanocomposites in the presence of compatibilisers and stabilizing system. Polym Degrad Stabil 95(12): 2547-2554.
- Moghri, M, Garmabi H, Zanjanijam AL (2018) Prediction of barrier properties of HDPE/PA-6/nano clay composites by response surface approach: effects of compatibilizer type and the contents of nanoclay, PA6 and compatibilizer. Polym Bull 75(7): 2751-2767.
- Zhou S, Wang J, Wang S, Ma X, Huang J, et al. (2018) Facile preparation of multiscale graphene-basalt fiber reinforcements and their enhanced mechanical and tribological properties for polyamide 6 composites. Mater Chem Phys 217: 315-322.
- Silva GDASD, Almeida JRM (2022) Mechanical properties and morphology of HDPE/PA12 blends compatibilized with HDPE-alt-MAH. Polym Polym Compos 30.
- Fang Z, Xu Y, Tong L (2007) Effect of clay on the morphology of binary blends of polyamide 6 with high density polyethylene and HDPE-graft-acrylic acid. Polym Eng Sci 47(5): 551-559.
- Das V, Kumar V, Singh A, Gautam SS, Pandey AK (2012) Compatibilization efficacy of LLDPE-g-MA on mechanical, thermal, morphological and water absorption properties of nylon-6/LLDPE blends. J Polym-Plast Technol 51(5): 446-454.
- Yasir QG, Song M, Abid U (2020) Permeation characterization and modeling of polyethylene/clay nanocomposites for packaging. Polym Bull 77(7): 3749-3765.
- Sharifi-Sanjani N (2010) Reactive compatibilization in compounding of polymers. Tehran university press, Tehran, Iran, p. 84.
- Yeh JT, Jyan CF (1998) Effects of Polyethylenes on the morphology, barrier, and impact properties of polyethylene/modified polyamide blends. Polym Eng Sci 38(9): 1482-1490.
- Ghanta TS, Aparna S, Verma N, Purnima D (2020) Review on nano-and micro filler-based polyamide 6 hybrid composite: Effect on mechanical properties and morphology. Polym Eng Sci 60(8): 1717-1759.
- Erdmann E, Acosta D, Pita VJ, Monasterio FE, Carrera MC, et al. (2010) Effect of the organoclay preparation on the extent of intercalation/exfoliation and barrier properties of polyethylene/PA6/montmorillonite nanocomposites. J Appl Polym Sci 118(4): 2467-2474.
- Liu T, Wang Q, Xie Y, Lee S, Wu Q (2014) Effects of use of coupling agents on the properties of microfibrillar composite based on high-density polyethylene and polyamide-6. Polym Bull 71(3): 685-703.
- Wei X, Lu Y, Huang L (2011) Mechanical properties and morphology of UHMWPE/PC/HDPE-g-MAH blends. Polym-Plast Technol 50(2): 190-195.
- Li LP, Yin B, Zhou Y, Gong L, Yang MB, et al. (2012) Characterization of PA6/EPDM-g-MA/HDPE ternary blends: The role of core-shell structure. Polymer 53(14): 3043-3051.
- Li S C, Tao L (2010) Melt rheological and thermos responsive shape memory properties of HDPE/PA6/POE-g-MAH blends. Polym-Plast Technol Eng 49(2): 218-222.
- Sharif-Pakdaman A, Morshedian J, Jahani Y (2013) Effect of organoclay and silane grafting of polyethylene on morphology, barrierity, and rheological properties of HDPE/PA6 blends. J Appl Polym Sci 127(2): 1211-1220.
- Pircheraghi G, Nazockdast H, Salehi MM (2011) The effects of chemical bonding of nanoclay surface modifier and compatibilizer on microstructure development and rheological properties of PP/PP-g-MA/diamine modified nanoclay. Polym-Plast Technol Eng 50(11): 1109-1117.
- Aubry T (2019) An overview on clay-mediated compatibilization of polyethylene/polyamide blends with droplet morphology. Appl Clay Sci 175: 184-189.
- Salmah H, Lim BY, Teh PL (2012) Melt rheological behavior and thermal properties of low-density polyethylene/palm kernel shell composites: effect of polyethylene acrylic acid. Int J Polym Mater 61(14): 1091-1101.
- Kazemi A, Khorasani SN, Dinari M, Khalili S (2021) Mechanical and barrier properties of LLDPE/TPS/OMMT packaging film in the presence of POE-g-IA or POE-g-MA. J Polym Res 28(4): 1-12.
- Scaffaro R, La Mantia FP, Canfora L, Polacco G, Filippi S, et al. (2003) Reactive compatibilization of PA6/LDPE blends with an ethylene-acrylic acid copolymer and a low molar mass bisoxazoline. Polym 44(22): 6951-6957.
- Aparna S, Purnima D, Adusumalli RB (2017) Review on various compatibilizers and its effect on mechanical properties of compatibilized nylon blends. Polym Plast Technol Eng 56: 617-634.
- Filippone G, Dintcheva NTZ, Acierno D, La Mantia FP (2008) The role of organoclay in promoting co-continuous morphology in high-density poly(ethylene)/poly(amide) 6 blends. Polym 49(5): 1312-1322.
- Hemmati M, Narimani A, Shariatpanahi H, Fereidoon A, Ghorbanzadeh AM (2011) Study on morphology, rheology and mechanical properties of thermoplastic elastomer polyolefin (TPO)/carbon nanotube nanocomposites with reference to the effect of polypropylene-grafted-maleic anhydride (PP-g-MA) as a compatibilizer. Int J Polym Mater 66(6): 384-397.
- Ataeefard M, Moradian S (2011) Polypropylene/organoclay nanocomposites: effects of clay content on properties. Polym-Plast Technol Eng 50(7): 732-739.
- Siddique SA (2020) Processing, structure and thermo-mechanical properties of reclaimed nanoclay, and its application in polyamide 6 and low-density polyethylene nanocomposites. Diss 2020
- Filippi S, Chiono V, Polacco G, Paci M, Minkova LI, (2022) Reactive compatibilizer precursors for LDPE/PA6 blends, 1. Ethylene/acrylic acid copolymers. Macromol Chem Phys 203: 1512-1525.
- Jiang C, Filippi S, Magagnini P (2003) Reactive compatibilizer precursors for LDPE/PA6 blends. II: maleic anhydride grafted polyethylenes Polym 44(8): 2411-2422.
- Yeh JT, Huang SS, Yao WH (2000) Gasoline permeation resistance of containers of polyethylene, polyethylene/modified polyamide and polyethylene/blends of modified polyamide and ethylene vinyl alcohol. Macromol Mater Eng 287(8): 532-538.
© 2023 Azita Alipour. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






