- Submissions

Full Text
Research & Development in Material Science
Kinetic Studies of the Oxidation of Primary Acyclic and Secondary Cyclic Perfumery alcohols using N- Bromosuccinimide in Alkaline Medium
DV Prabhu1*, Himanshu Gupta2, Freddy H Havaldar1 and Harichandra A Parbat2
1Department of Chemistry, Wilson College, India
2Department of Chemistry, St. Xavier’s College, India
*Corresponding author:DV Prabhu, Department of Chemistry, Wilson College, India
Submission: June 14, 2023;Published: June 22, 2023

ISSN: 2576-8840 Volume 19 Issue 1
Abstract
India has a large perfumery industry and a worldwide market for perfumes, fragrances and cosmetic formulations. Many of these perfumery chemicals are extracted from Indian plants and hence the study of perfumery alcohols becomes relevant. The quantitative aspects of the oxidation of alcohols to the corresponding carbonyl compounds has been extensively reported. But literature survey shows relatively few reports of the kinetic and thermodynamic studies of oxidation of perfumery alcohols. This paper reports the kinetics of the controlled oxidation of 1) primary acyclic alcohols, Geraniol, Nerol and Citronellol and 2) secondary cyclic alcohols, Cyclopentanol and Cyclohexanol using N- Bromosuccinimde in alkaline medium. The oxidation was carried out under first order kinetic conditions with respect to the organic oxidant i.e. [oxidant] << alc.].
The effects of alcohol and oxidant concentrations, ionic strength and temperature on the rates of oxidation of alcohols has been studied. The thermodynamic activation parameters were determined from the change of oxidation rate of alcohol with temperature and interpreted in terms of the rection mechanism proposed for the oxidation.
Keywords:Primary acyclic alcohols; Secondary cyclic alcohols; Oxidation; N-Bromosuccinimide; Kinetics; Ionic strength; Thermodynamic activation parameters; Entropy of activation; Reaction mechanism
Introduction
The quantitative aspects of the oxidation of alcohols has been extensively reported [1-6] but there are few reports of the kinetic studies of oxidation of perfumery alcohols [7-10]. We have earlier reported the kinetics of oxidation of some industrially important alcohols using organic and inorganic oxidants [11-14].
This study deals with the first order kinetics of the controlled oxidation of primary acyclic alcohols, Geraniol, Nerol and Citronellol and secondary cyclic alcohols, Cyclopentanol and Cyclohexanol by N-Bromosuccinimide in alkaline medium. The effects of alcohol and oxidant concentrations, ionic strength and temperature on the oxidation rates of the alcohols under study have been studied. On the basis of the experimental data obtained suitable reaction mechanisms have been suggested for the oxidation of perfumery alcohols.
Figure 1:Cv and Ci concentration near critical point [4].

Materials and Methods
The perfumery alcohols were obtained from S H Kelkar & Co., Mumbai and used after distillation. Analytical Grade N-Bromosuccinimide and chemicals were used to study the oxidation of alcohols. The requisite solutions of alcohol and oxidant ([Oxidant ] << [alc.]) were allowed to equilibrate in a previously adjusted thermostat (accuracy +/- 0.1 °C) for about 15 minutes. After the temperature equilibrium was attained, the solutions were quickly mixed to initiate the reaction. Aliquots of the reaction mixture were withdrawn at regular time intervals during the course of the reaction, the reaction was arrested using ice and the unreacted oxidant was estimated iodometrically. The rate constants (k) were determined from the straight line graphs of log (unreacted oxidant) v/s time.
The effect of ionic strength on oxidation rate was determined by using Analytical Grade K2SO4 in dilute solution. From the effect of temperature on oxidation rate of alcohol, the thermodynamic activation parameters were evaluated and interpreted in terms of the reaction mechanism suggested for the oxidation.
Figure 2:Schematic illustration of growing ingot and temperature gradients for the NOC method [3,5].

Results and Discussion
The primary acyclic alcohols, Geraniol, Nerol and Citronellol were oxidized to the corresponding aldehydes by N-Bromosuccinimide [NBS] in alkaline medium
The rate constant data for oxidation of these primary acyclic alcohols is given in Table 1.
Table 1:Rate constant data for oxidation of primary acyclic alcohols by N-Bromosuccinimide in alkaline medium at 303K. [NaOH] = 0.05mol dm-3.
Citronellol
The oxidation rates of alcohols follow the sequence, Nerol > Geraniol > Citronellol which is consistent with regard to their steric hindrance effects on their oxidation (Table 1)
Geraniol
For all the primary acyclic alcohols studied, the oxidation rate increased with [alc.] but decreased with [NBS] (Table1).
Reaction mechanism of oxidation of primary acyclic alcohols by N-Bromosuccinimide in alkaline medium
In alkaline medium, the oxidation proceeds via the formation of a complex between the active species of the oxidant and the substrate followed by the decomposition of the complex in a slow rate determining step to yield the aldehyde.
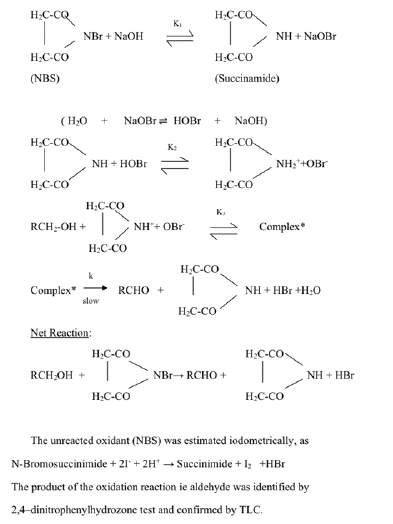
(In the case of a secondary alcohol RR’CHOH, the corresponding ketone, RR’C=O is produced).
Effect of ionic strength on the oxidation rates of primary acyclic alcohols by N-Bromosuccinimide in alkaline medium
Table 2:Effect of ionic strength on the rates of oxidation of primary acyclic alcohols by N-Bromosuccinimide in alkaline medium.
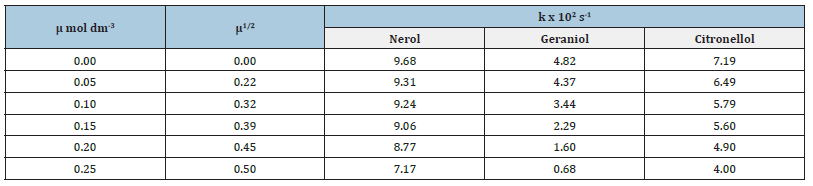
Analar Grade K2SO4 was used in the range μ=0.05-0.25mol dm-3 to study the effect of ionic strength on oxidation rates of primary acyclic alcohols (Table 2).
The graphs of log k v/s μ1/2 were found to be straight lines parallel to the μ1/2 axis indicating that ionic strength has no effect on the oxidation rate of alcohols as borne out by the reaction mechanism suggested. This observation is in accordance with the Bjerrum-Bronsted equation, logk=logk0+1.02 ZAZB μ1/2 where ZA and ZB are the valencies of the ions involved in the oxidation reaction.
Effect of temperature on the oxidation rates of primary acyclic alcohols by N-Bromosuccinimide in alkaline medium.
The reaction was carried out in the temperature range 303-318K and the rate constants were determined from the Arrhenius plots of logk v/s T-1. From the variation of oxidation rate with temperature, the thermodynamic activation parameters were determined (Table 3) and interpreted in terms of the reaction mechanism proposed.
Table 3:Thermodynamic activation parameters of the oxidation of Primary acyclic alcohols by N-Bromosuccinimide in
alkaline medium.
[alc.]= 0.1mol dm-3, [NaOH]=0.05mol dm-3, [NBS]= 2.5x10-3mol dm-3
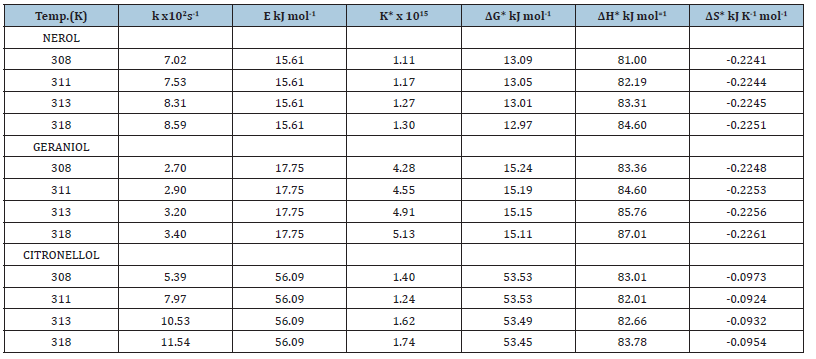
For all the alcohols studied, the oxidation rate increased with temperature. The negative values of entropy of activation (ΔS*) indicate a decrease in the degrees of freedom due to the formation of a rigid activated complex during the course of the reaction. This results in the orientation of the solvent molecules around the activated complex and consequently the entropy of the reacting system [15,16].
The secondary cyclic alcohols, Cyclopentanol and Cyclohexanol were oxidized to the corresponding ketones by N-Bromosuccinimide in alkaline medium.
Table 4 gives the rate constant data of these secondary cyclic alcohols.
Table 4:Rate constant data of the oxidation of secondary cyclic alcohols by N-Bromosuccinimide in alkaline medium at 303K.

The oxidation rates follow the sequence, Cyclopentanol > Cyclohexanol.
Cyclopentanol
Cyclohexanol 5, 7 and 8 membered rings in cyclic alcohols are more reactive than 6 -membered rings as in Cyclohexanol [17-19]; (Table 4). 6-membered rings have least strain and hence are least susceptible to oxidation. For both cyclic alcohols under study, the oxidation rate is proportional to alcohol concentration but inversely proportional to NBS concentration (Table 4).
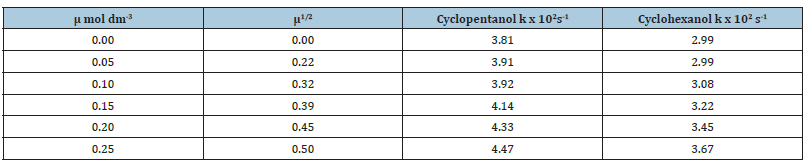
Effect of ionic strength on the oxidation rates of secondary cyclic alcohols using N-Bromosuccinimide in alkaline medium
Analytical grade K2SO4 was used in the range μ=0.05-0.25mol dm-3. to study the effect of ionic strength on the rates of oxidation of secondary cyclic alcohols (Table 5). The graphs of logk v/s μ1/2 were found to be straight lines parallel to the μ1/2 axis indicating that the oxidation is independent of ionic strength.
Table 5:Effect of ionic strength on the oxidation of cyclic secondary alcohols by N-Bromosuccinimide in alkaline
medium.
[alc.]=0.1mol dm-3, [NaOH]=0.05mol dm-3, [NBS]=2.5 x10-3 mol dm-3
Effect of temperature on oxidation rates of secondary cyclic alcohols by N-Bromosuccinimide in alkaline medium
Table 6:Thermodynamic activation parameters of the oxidation of secondary cyclic alcohols by N-Bromosuccinimide in
alkaline medium.
[alc.]=0.1mol dm-2, [NaOH]=0.05mol dm-3, [NBS]= 2.5x10-3mol dm-3
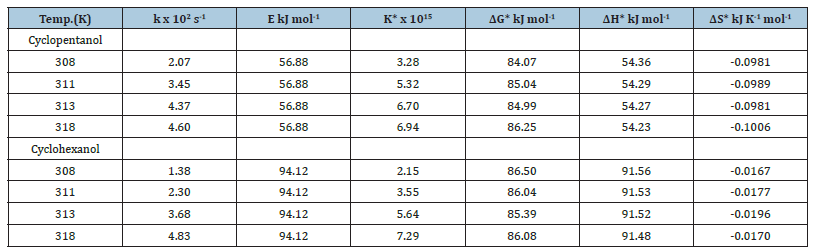
The oxidation of secondary cyclic alcohols, Cyclopentanol and Cyclohexanol was carried out in the temperature range 308- 318K and from the change of oxidation rate with temperature, the thermodynamic activation parameters were determined (Table 6).
Conclusion
The oxidation rate increases with alcohol concentration but deceases with oxidant concentration for all the perfumery alcohols investigated
Ionic strength has no effect on the rates of oxidation of the alcohols indicating the involvement of a non-ionic species (alcohol) in the oxidation process.
The oxidation is accompanied by decrease in entropy of activation due to the orientation of solvent molecules around the rigid activated complex formed as an unstable intermediate during the reaction.
The constant values of entropy of activation at different temperatures for a given alcohol indicate that the site of oxidation i.e.-OH bond is the same at all temperatures.
References
- Corey EJ , Suggs JW (1975) Pyridium chlorochromate: An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds. Tetrahedron Letters 16(31): 2647-2650.
- Corey EJ, Boyer BL (1978) Tetrahedron Letters 19: 240.
- Corey EJ, Schmidt G (1978) Tetrahedron Letters 20: 299.
- Gunasekaran S, Venkatsubramanian N (1983) Oxidation of phenylmethylcarbinols by bromamine-T. Proc. Indian Acad Sc (Chem Sc) 92(1): 107-112.
- Lee SV, Madin A, Trost BM (Eds.) (1991) Comprehensive Organic Chemistry, Pergamon Press, Oxford, England, 7: 251.
- Magdziak D, Rodriquez AA, Van De Water RW, Petus TR (2002) Regioselective oxidation of phenols to o-quinones with o-iodobenzoic acid(IBX). Organic Letters 4(2): 285-288.
- Hudlicky M (1990) Oxidations in Organic Chemistry, American Chemical Society Monographs, p.186.
- Choudhary PK, Sharma PK, Banerjee KK (2000) Intl J Chemical Kinetics 31: 469.
- Das Asim K (2000) J Indian Chem Soc 77: 225.
- Nandibewoor ST, et al. (2000) J Indian Chem Soc 77: 363.
- Srivastava Sheila (2008) Asian J Chem 20: 4776.
- Prabhu DV, Parbat HA, Tandel MA (2011) A Kinetic approach to the oxidation of some perfumery alcohols by N-Bromosuccinimide in alkaline medium. Asian J Chemistry 23(12): 5495-5498.
- Prabhu DV, Parbat HA, Tandel MA (2012) Catalytic effect of transition metal ions on the oxidation of fragrance and cosmetic alcohol. Asian J Chem 24(12): 5687-5690.
- Prabhu DV, Tandel MA, Parbat HA (2014) Kinetic studies of the transition metal ion catalyzed oxidation of some fragrance alcohols. Asian Journal of Chemistry 26(19): 6669-6673.
- Harichandra A, Parbat Prabhu DV, Chetana M Rana (2021) A comparative study of the transition metal ion catalyzed oxidaton of perfumery alcohols by kinetic methods. GP Globalize Research Journal of Chemistry 5(1): 72-80.
- Prabhu DV, Himanshu D, Gupta Freddy Havaldar (2021) Kinetic and thermodynamic studies of the controlled oxidation of Cinnamyl alcohol by K2S2O8 in acidic medium. Asian J of Org and Medicinal Chemistry 7(1): 104-106.
- Eichhorn GL, Trachtenberg IM (1954) J Am Chem Soc 76: 5184.
- Amiss ES (1949) Kinetics of Chemical Change in Solution. The Macmillan Co., New York, USA, p.108-110.
- Brown HC, Herstein H (1952) J Am Chem Soc 74: 2929.
- Kuivila HG, Becker BT (1952) J Am Chem Soc 74: 5329.
- Brown HC, Fletcher RS, Johannesen RB (1957) J Am Chem Soc 79: 212.
- Prabhu DV (2008) Kinetic and reaction mechanism of the oxidation of some cyclic alcohols in the micellar phase using Chloramine T in alkaline medium. Oriental Journal of Chemistry 24(1):163-166.
© 2023 DV Prabhu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






