- Submissions

Full Text
Research & Development in Material Science
Synthetic Lonecreekite (NH4) Al0.59Fe0.41(SO4)2.12H2O
Vahobjon Sabirov*
M Auezov South-Kazakhstan State University, Republic of Kazakhstan
*Corresponding author: Vahobjon sabirov, M Auezov South-Kazakhstan State University, Republic of Kazakhstan
Submission: April 04, 2023;Published: April 19, 2023

ISSN: 2576-8840 Volume 18 Issue 5
Summary
The crystal structure of the synthetic mineral lonecreekite, (NH4)Al0.59Fe0.41(SO4)2.12H2O (I) has been determined from single-crystal X-ray diffraction. The crystal structure contains Centro symmetrical octahedral [M(H2O)6]3+ (M = Al3+ and Fe3+) and [(NH4)+(H2O)6] cations as well as (SO4)2- anions. The M3+- OH2 distance is between the analogical distances for Al3+ and Fe3+ cations. The M cation and N atom are placed on inversion 3-fold axis (4(a) and 4(b) special positions). The sulfate anion is distributed in two mutually pseudo reversed positions with the relative occupancies 0.91 and 0.09. The S and two mutually opposite posioned O atoms of the disordered sulfate anion are placed on a 3-fold axis (8(c) special position). The H atoms of the (NH4)+ group are not located, but the geometry of the eight short contacts of the N atom corresponds to the H-bonds and shows that cation is oriented in two positions related to each other by an inversion center.
Keywords:Calcium carbonate; Synthesis; Albumin; Bio mineralization; Simulation
Introduction
Double sulfates described by simplest common formula AB(SO4)2.12H2O, where A = Na, K, Rb, Ce, or Tl, NH4+, CH3NH3+ etc. and B = Al, Cr, Ti, Mn, V, Fe(3+), Co(3+), Ga(3+) etc., are known as alums [1]. There are three types of alums called as α-, β- and γ-alums [2]. Although alums have enoughly simple chemical composition, owing to disorder in the arrangement of the structural units in the crystal structures, they reveal some anomalous optical properties. Birefringence, or double refraction, is one of them. Kinetic ordering of cations in solid solutions of alums showing anomalous birefringence was studied by means of single-crystal X-ray diffraction way for KAl0.6Cr0.4(SO4)2.12H2O, K0.45(NH4)0.55Al(SO4)2.12H2O [3] and KAl0.95Cr0.05(SO4).12H2O [4]. Paramagnetic-resonance spectroscopy study of the Ti3+-substituted alums shows, that the distortion of the position of the (SO4)2- group and the perceptible difference between ionic radius of the Ti?3+ and Al?3+ ions may be main cause of the reducing of the local crystal field symmetry [5]. According to the X-ray and neutron diffraction study, there are several types of distortions in the alum crystals. The orientational disorder of the sulfate oxygen atoms are often observed type of disorder. That was especially studied for several alums [6]. Disorder of the ammonium cation and as well as sulfate group has been observed in the crystal structure of ammonium aluminium alum [7]. In crystal structures of the sodium aluminium alum [8], rubidium chromium alum [9] and ammonium chromium alum [10] the disorder is absent.
The substitutional disorder of the trivalent cation in alum crystals is not widely known. The crystal structure of KAl0.6Cr0.4(SO4)2.12H2O [3] and KAl0.95Cr0.05(SO4).12H2O [4] are rare samples of solid solution of alum. Natural mineral lonecreekite, (NH4)Fe0.75Al0.25(SO4)2.12H2O [11], is first the solid solution of alum containing two different M3+cations with perceptible difference in an ionic radius. The presence of two different M3+ metal cations generates the additional distortions in the three-dimensional crystal structure of the alum and may lead to different interesting physical properties. For this reason, the new synthetic mineral lonecreekite (NH4)Al0.59Fe0.41(SO4)2.12H2O (I) was obtained and studied by a single-crystal X-ray diffraction. The purpose of the present paper is to define the influence of the mutual substitution of Al3+ and Fe3+ ions on the geometrical parameters of the [M(H2O)6]3+ octahedron and also to discuss the problems arising by the refinement of the solid solution crystal structure.
Methodology of the Experiment
Crystals of Fe, Al-alum, (NH4)Fe0.40Al0.60(SO4).12H2O (I) was obtained by addition of aluminium alum to sulfuric acid solution (pH<4) containing ammonium and iron atoms. The solution was slowly heated to 350K and then it was left for evaporation at room temperature. After one days, small size colorless octahedral crystals appears on the bottom of the glass. The crystals were isolated from solution, dried and chosen by sizes for single X-ray diffraction experiments. At the further evaporation of the solution, the octahedral crystals take up the hexagonal antiprism form. Thus, an inversion axis of symmetry appears in the habitus of the alum crystals. The crystal density was determined using the flotation method in the inert organic liquid.
Refinement
All H atoms, except H atoms of ammonium group, were located in difference Fourier maps and refined isotopically. The O-H distance are in the range 0.67-0.89 (3) and H-O-H bond angles equal to 102 (3) and 111 (4)o. In all stages of the refinement one weak peak permanently appeared on the difference Fourier map near N atom. However, it was not identified as atom of hydrogen. The relatively occupancies of Al and Fe atoms also O1 and O2 atoms were refined using free variables. The relative occupancies for atoms Al and Fe respectively equal to 0.59 (5) and 0.41 (5). Obtained X-ray diffraction results agree with results of massspectrometry measurement within experimental error. The SO4 group is distributed in two mutually pseudo reversed orientations with relatively occupancies 0.907 (6) (0.91) and 0.093 (6) (0.09) respectively for “normal” orientation (for O1 and O2 atoms) and “pseudo reversed” orientation (for O1A and O2A atoms). The O1A and O2A atoms of the sulfate group have been located in the difference Fourier maps and both were sited in a general position. Because of this, for O1A atom the coordinates of analogical atom in the crystal structure of the ammonium-alum were used. The anisotropic displacement parameters of the O1A O2A atoms were very large and the sulfate group in “pseudo reversed” orientation had very distorted geometry. The anisotropic displacement parameters of the O1A and O2A atoms and also geometry of the SO4 tetrahedron were corrected using SIMU and SADI restriction instructions. Disorders in the lonecreekite effect on the collected diffraction data and convergence of the calculated and experimental scattering factors. The 14 reflections forbidden in the space group Pa3−were observed.
Computing details
Data collection: CrysAlisPro [12]; cell refinement: CrysAlisPro; data reduction: CrysAlisPro; program(s) used to solve structure: SHELXS97 [13]; program(s) used to refine structure: SHELXL97 [13]; molecular graphics: SHELXTL [13] and Mercury [14]; software used to prepare material for publication: SHELXTL.
Results and Discussion
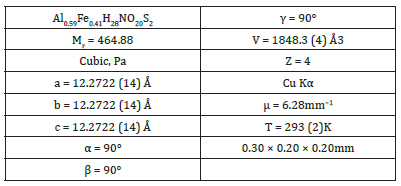
The parameters of the unit cell of I (12.2722(14) Å) is appreciable more than that for (NH4)Al(SO4)2.12H2O (12.242 (1)Å) [7] and less than that for (NH4)Fe0.75Al0.25(SO4)2.12H2O (12.302 Å) [11] (all single-crystal X-ray diffraction experiments are performed at room temperature). Crystal ionic radius equal to 0.50Å and 0.64Å [15] respectively for Al3+ and Fe3+ cations. Increasing of the share of the Fe3+ ion in aluminum alum tend to increasing of the crystal parameter a and the cell volume V. The crystal structure of I is isomorphous to the crystal structures of the others alums: the trivalent metal ion M (Al3+ and Fe3+) is placed on inversion 3-fold axis (4(a) special position) and are coordinated in a regular octahedral geometry by six water molecules (Table 1 & Figure 1). In the [M(H2O)6]3+ octahedron, the M3+-OH2 distance equal to 1.9235 (14) Å, which is appreciably longer than the Al-OH2 bond distance in the structures of ammonium alum 1.883 (1) Å [7], sodium alum 1.881 (1) [8] and potassium alum 1.908 (8) Å [16]. At the same time, the M-O distance is shorter than the sum of the ionic radii of the Fe3+ cation and oxygen atom (2.00Å) [15].
Figure 1:The [M(H2O)6]+ and [(H2O)6]+ cations (H atoms of the (NH)4
+ group were not positioned) also (SO
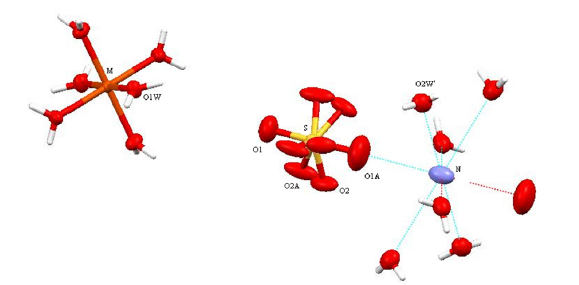
Table 1:Crystallographic data and structure refinement summary for crystal I.
The difference in the geometrical parameters of the coordination polyhedra [Fe(H2O)6]3+ and [Al(H2O)6]3+ causes the local distortions in the crystal lattice. Other type of the disorder is caused by the stochastic distribution of the iron(3+) and aluminium cations in the crystal. In I, the sulfate group is disordered in two orientations with the relative occupancies 0.91 and 0.09. The S and mutually opposite placed O1 and O1A atoms are on a threefold axis (8(c) special position). The O2 and O2A atoms occupy a general position. The O atoms in the more occupied orientation form almost regular tetrahedron around S atom, but the O atoms from poorly populated positions form distorted tetrahedron. The H-bonds in I is similar in design to that in alum crystals with disordered sulfate group (Figure 2 & Table 2). The complex cation [M(H2O)6]3+ is linked with two different (SO4)2- anions through intermolecular O1W-H11···O2 (-z+1/2, x-1/2) and O1W-H11···O2A (-y+1/2, z-1/2) H-bonds. The second water molecule is linked with that complex cation through O1W-H12···O2W H-bond (Table 3 & 4).
Figure 2:Packing diagram of I along the c axis (the broken lines show hydrogen bonds). Hydrogen atoms of the ammonium group are not located.
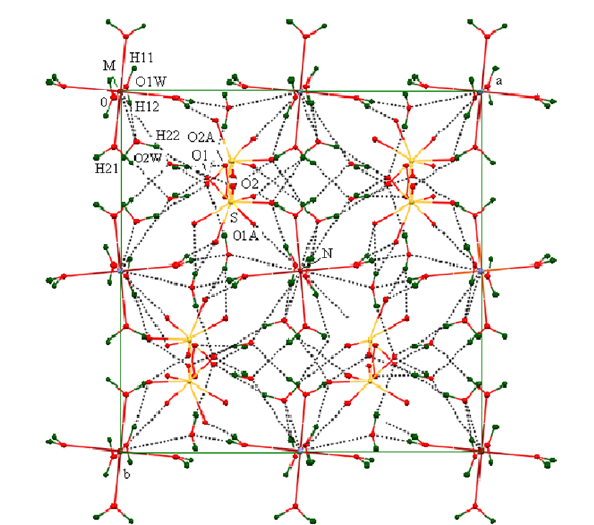
Table 2:
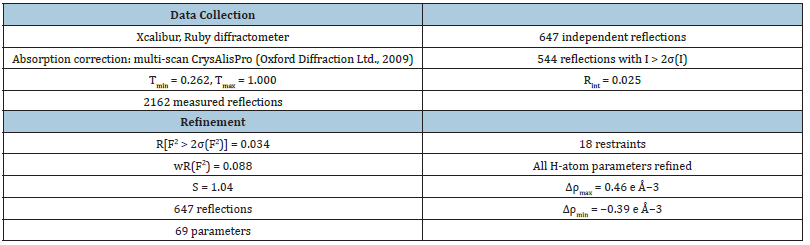
Table 3:Selected geometric parameters (Å,°).
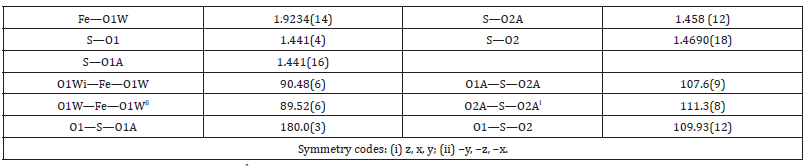
Table 4:Hydrogen-bond geometry (Å,°).
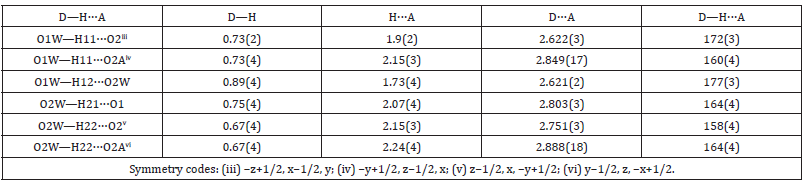
The N atom of the (NH4)+ group lays on inversion 3-fold axis (4(b) special position) at 1/2 1/2 1/2. Analogical distribution of the monovalent cation was observed also in the crystal structures of the sodium alum [8] and ammonium chromium alum [10]. In the crystal structure of ammonium aluminum alum, the N atom is distributed on general position 24(d) around an inversion centre at two positions with equal probability [7]. The nitrogen atom N has eight Centro symmetrically related short contacts correspond to the H-bonds: two N···O1A (2.629 (10) Å) and six N···O2W (3.034 (2) Å). They reveal that the NH4+ cation is oriented in two Centro symmetrically related positions. Symmetrically positioned around the N atom two O1A atoms and six O2W atoms form two Centro symmetrically related tetrahedron (Figure 1). The angles O1A···N···O2W 104.4 (3) and O2W···N···O2W 114.0° close to tetrahedral. The ammonium cation in the crystal structure of the ammonium alum [7] also forms analogical H-bonds.
Conclusion
Thus, performed X-ray diffraction study of the synthetic lonecreekite reveals, that in the crystal structure of the solid solution of alum containing comparable amounts of Al and Fe, the main structural behaviours of the crystal structure of the alum are remain, but the unit cell and the cation octahedra [M(H2O)6]3+ parameters are distinguished. Some infringement of the standard intermolecular distances is observed. Among the X-ray diffraction data several forbidden in the space group Pa [3] reflections are present.
Acknowledgements
Author thank colleagues from Bioorganic Chemistry Institute of the Academy of Sciences of Uzbekistan for X-ray diffraction experimental supporting in this studying.
References
- Greenwood NN, Earnshaw A (1997) Chemistry of the elements. (2nd edn), Butterworth-Heinemann, Oxford, UK.
- Lipson H (1935) The relation between the alum structures. Proc Roy Soc A 151(873): 347-356.
- Shtukenberg AG, Euler H, Kirfel A (2007) Symmetry reduction and cation ordering in alum solid solutions. Z Kristallogr. 222(2): 73-82.
- Rozhdestvensakaya IV, Frank-Kamenetskaya OV, Shtukenberg AG, Bannova II (2001) Triclinic structure of birefringent crystals of K(Al95Cr0.05)(SO4)2⋅12H2O. J Struct Chem (Russ) 42: 628-638.
- Dionne GF, MacKinnon JA (1968) The mixed state in superconducting thin films. Physical Review 172(2): 325-330.
- Nyburg SC, Steed JW, Aleksovska S, Petruševski VM (2000) Structure of the alums I on the sulfate group disorder in the α-alums. Acta Cryst B 56(2): 204-209.
- Abdeen AM, Will G, Schafer W, Kirfel A, Bargouth MO, et al. (1981) X-Ray and neutron diffraction study of alums. Z Kristallogr 157: 147-166.
- Cromer DT, Kay MI, Larson AC (1967) Refinement of the alum structures II X-ray and neutron diffraction of NaAl(SO4)2.12H2O, γ Acta Cryst 22(2): 182-187.
- Figgis BN, Reynolds PhA, Sobolev AN (2000) The structures of the α-alums RbCr(SO4)212H2O and CsCr(SeO4)2·12H2O at 293 and 12 K. Acta Cryst C 56(7): 731-734.
- Rempfer N, Lerner HW, Bolte M (2004) The ammonium chromium(III) alum NH4Cr(SO4)212H2O. Acta Cryst E 60(7): i80-i81.
- Martini JEJ (1983) Lonecreekite, sabieite, and clairite, new secondary ammonium ferric-iron sulfates from Lone Creek Fall cave, near Sabie, Eastern Transvaal. Ann Geolm Surv S Africa 17: 29-34.
- Oxford Diffraction (2009) Growth and characterization of non-linear optical material. Crys Alis Pro 10(12).
- Sheldrick GM (2008) A short history of SHELX. Acta Cryst A 64: 112-122.
- Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, et al. (2006) Mercury: Visualization and analysis of crystal structures. J Appl Cryst 39: 453-457.
- Shannon RD, Prewitt CT (1969) Enhanced visible-light-sensitive two-step overall water-splitting based on band structure controls of titanium dioxide and strontium titanate. Acta Cryst B 5(1): 925-946.
- Sheldrick GM (1990) Phase annealing in SHELX-90: Direct methods for larger structures. Acta Cryst A 46(6): 467-473.
© 2023 Vahobjon Sabirov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






