- Submissions

Full Text
Research & Development in Material Science
Biomineralization of Calcium Carbonate in Water Solutions with Egg Wite Albumine
Zakharov NA1*, Koval EM1, Goeva LV1, Shelichov EV2, Aliev AD3, Kiselev MR3, Маtvеев VV3 and Zakharova TV4
1Kurnakov Institute of Gentral and Inorganic Chemistry, Moscow, Russia
2NITU, Lininsky, Moscow, Russia
3Frumkin Institute of Physical Chemistry and Electrochemistry, Moscow, Russia
4Russian University of Transport, MIIT, Moscow, Russia
*Corresponding author: Zakharov NA, Kurnakov Institute of Gentral and Inorganic Chemistry, Moscow, Russia
Submission: March 20, 2023;Published: April 14, 2023

ISSN: 2576-8840 Volume 18 Issue 3
Abstract
Topical is the trend of investigation, that connected with synthesis of perspective for medical practice materials on the base of calcium carbonate (CaCO3, CC). Among a number of polymorphous modifications of calcium carbonates (crystalline anhydrous calcite, aragonite, vaterite; hydrated - mono- (CaCO3·H2O) hexahydrate- (CaCO3·6H2O); amorphous phase CC) the greatest interest for application as materials for targeted delivery of medical preparations presents vaterite. The results of bio mineralization simulation of calcium carbonate with participation of egg white albumine (ALB) in conditions precipitation from water solutions in water system CaCl2-Na2CO3-ALB-Н2О are considered and carried out assessment effect of ALB content in solution on formation in composition of synthesis products of phases Calcite (C), Aragonite (A) and Vaterite (V) were realized. The synthesis products identified by methods of physical and chemical analysis (chemical, X-Ray, infrared spectral analysis, scanning- and translucent electron microscopy) and fundamental interactions composition - synthesis conditions - structure - properties for prepared synthesis products were estimated. Developed and realized approaches for directed synthesis materials with regulated in course of synthesis and following processing properties on the base of calcium carbonate for medical purposes and variants of applications in medical practice synthesized materials were considered. .
Keywords:Calcium carbonate; Synthesis; Albumin; Bio mineralization; Simulation
Mini Review
Table 1:Crystallographic characteristics of anhydrous polymorphous modifications of CC.
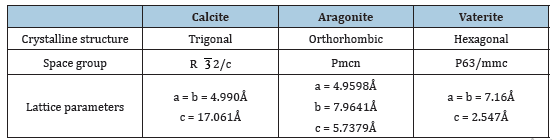
Calcium Carbonate (CC) has six known crystalline polymorphic modifications in nature. There are three anhydrous crystals (calcite, aragonite and vaterite) (Table 1), two hydrated phases of CC (mono- and hexahydrate CC: CaCO3·H2O, CaCO3·6H2O) and one hydrated amorphous (amorphous CC (ACC)) [1]..
CC is known as a cheap available commercial material [2]. It is widely used for various industrial applications [3]. Attention to the study of the features of CA crystallization is caused by widespread natural processes of calcification and bio mineralization: from bacteria to mammals [4]. Bio composites of various types such as organic/inorganic type of the bio minerals, include a large group of biological o bjects [5]. CC occupies a special place among biological minerals because it is a part of the bones and shells of animals. In this case, the inorganic material (CC) turns out to be associated with biopolymers [6].
The formation of a certain polymorphic modification of CС, which occurs during precipitation of CС crystals from aqueous solutions, can be influenced by such synthesis conditions as the type of precursors, the nature of their interaction during synthesis, as well as external influences - temperature, pressure, stirring rates and gas flow ( CO2) in solution. Evaluation of the effect of organic additives on the formation of AC from aqueous solutions is promising because it is effective to control the phase composition of synthesis products.
Synthesis of CC was carried out at 20 °C by pouring solutions of Na2CO3 and CaCl2 with subsequent stirring of the mixture. The addition of ALB was carried out dropwise with its aqueous solution in an amount corresponding to the preparation of synthesis products containing 4.9%, 9.8% and 19.6% w. % of ALB. The resulting mixture was settled. The synthesis product was filtered off.
The samples were identified using the methods of chemical and X-ray phase analysis, infrared spectroscopy (IR), scanning and transmission electron microscopy (SEM, TEM). The results of chemical and X-Ray analyzes, IR, indicate the formation of finegrained CC (Figure 1a & 1b). The amount of ALB introduced during the synthesis has a significant effect on the phase composition of the synthesis products (Figure 2a). As can be seen, the content of the most soluble and least stable phase of vaterite takes place at the highest content of ALB during the synthesis.
Figure 1:Diffractograms (a) and IR spectra (b) of the samples. Samples were synthesized by instantaneously pouring solutions of calcium chloride and sodium carbonate at 20 °C. Then, solutions of egg white albumin at various concentrations were gradually introduced by drop instillation into the samples: a – 4.9; 9.8; 14.7; 19.6 w. % ALB; b – (1) 4.9; (2) 9.8; (3) 14.7 w. % ALB.
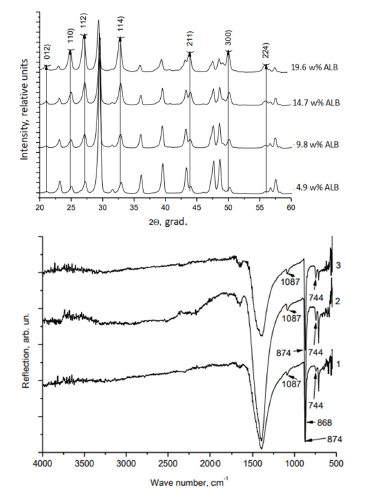
Figure 2:The content of various phases (a) and crystal sizes (b) of samples. Samples were synthesized by instantaneously pouring solutions of calcium chloride and sodium carbonate at 20 °C. Then, solutions of egg white albumin at various concentrations were gradually introduced by drop instillation into the samples.
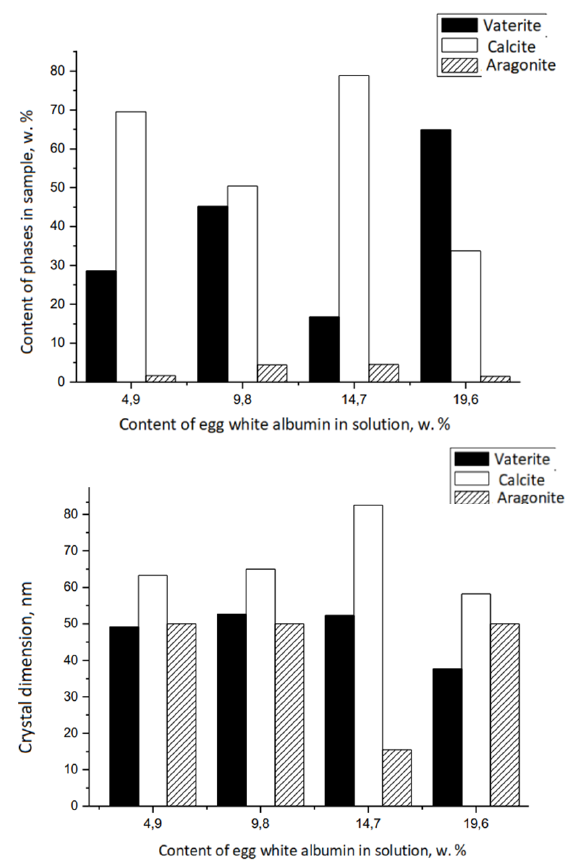
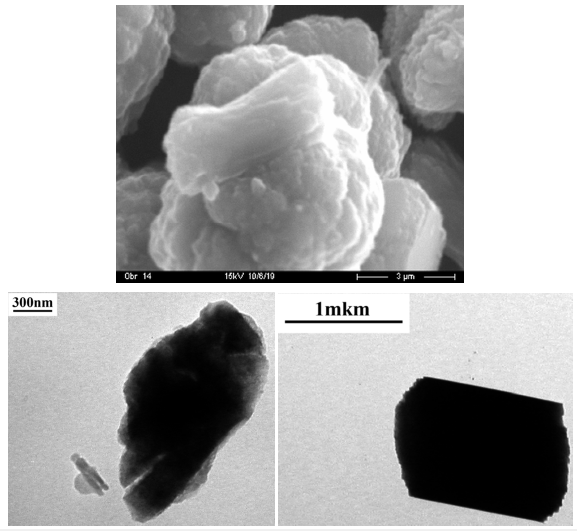
At the same time, the sizes of vaterite crystals (Figure 2b) are less affected by the ALB content during synthesis. The results of electron (SEM, TEM) microscopy (Figure 3a & 3b) confirm the data of X-ray diffractometry and IR spectroscopy and indicate that CC microcrystals (Figure 3a) are formed by aggregates of smaller CC nanocrystals (Figure 3b).
Figure 3:The results of SEM and TEM of samples synthesized with a content of 4.9 wt. % ALB.
Presented results
a) Will contribute to the development of methods for the
directed synthesis of CC during precipitation from aqueous
solutions in the CaCl2-Na2CO3-ALB-H2O system with controlled
parameters for the content of various phases (calcite, aragonite,
vaterite), particles of various sizes and a certain morphology in
the synthesis products;
b) Are of undoubted interest for the creation of biocompatible
drugs based on CC with different physicochemical and
biomedical characteristics.
References
- Kawano J, Shimobayashi N, Kitamura M, Shinoda K, Aikawa N (2002) Formation process of calcium carbonate from highly supersaturated solution. Journal of Crystal Growth 237: 419-423.
- Wang Y, Moo YX, Chen C, Gunawan P, Xu R (2010) Fast precipitation of uniform CaCO3 nanospheres and their transformation to hollow hydroxyapatite nanospheres. J Colloid Interf Sci 352: 393-400.
- Xiang L, Xiang Y, Wen Y, Wei F (2004) Formation of CaCO3 nanoparticles in the presence of terpineol. Materials Letters 58(6): 959-965.
- Mann S (2001) Bio mineralization principles and concepts in bioinorganic materials chemistry. Oxford University Press, UK, р. 401.
- Dujardin E, Mann S (2002) Bio-inspired materials chemistry. Advanced Materials 14(11): 1-14.
- Hunter KG (1996) Interface aspects of bio mineralization. Current Opinion Solid State & Materials Sci 1: 430-435.
© 2023 Zakharov NA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






