- Submissions

Full Text
Research & Development in Material Science
Experimental and Mathematical Modelling of Thermal Effects on Shale’s Permeability
Sarah Alenezi and Talal Al-Bazali*
Department of Petroleum Engineering, College of Engineering and Petroleum, Kuwait University, Kuwait
*Corresponding author: Talal Al-Bazali, Department of Petroleum Engineering, College of Engineering and Petroleum, Kuwait University, Kuwait
Submission: February 01, 2023;Published: February 20, 2023

ISSN: 2576-8840 Volume 18 Issue 3
Abstract
Pressure transmission tests and mathematical modelling were conducted to determine shale’s permeability under various temperatures (25 ˚C up to 300 ˚C). Results showed that for shales A, the permeability decreased as temperature increased from 25 ˚C to 100 ˚C and it increased thereafter (above 100 ˚C). As for shale B, the permeability decreased as temperature rose from 25 ˚C to 150 ˚C and then it increased as temperature progressed beyond 150 ˚C [1]. The behaviour of permeability of shale C was totally different as it increased for all temperatures. We believe that, among other factors, clay sites activation and pore dilation could, for most of the part, explain the behaviour of shale permeability for shales A, B & C under high temperatures. We think that extreme heat might activate clay sites and cause their detachment from shale surfaces. This thermal detachment of the clay platelets is demonstrated as a thermal swelling. Clay platelet detachment caused swelling that could narrow pore throats and bodies, decreasing the amount of void space in the network of pores. In addition, we believe that high temperature could enhance the size of pore throat of the shale which might cause an increase in its permeability. This process is referred to in the literature as ‘’pore dilation’’ [2-5]. Data also suggests that the amount of shale’s moisture between the grains is diminished by the application of heat. A reduction in water activity of all shales as temperature risen from 25 ˚C to 300 ˚C was observed.
Background
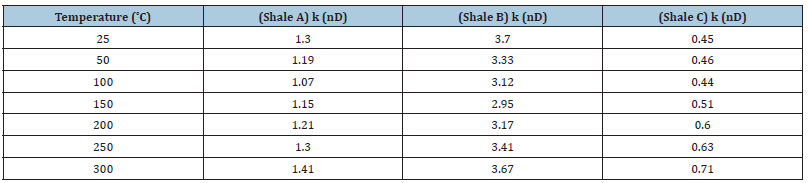
Figure 1:Estimated permeabilities for shales A, B and C under 25, 50, 100, 150, 200, 250, and 300 ˚C..
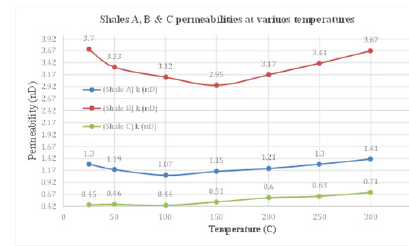
Permeability is defined as the ability of the rock sample to conduct fluids through it as shown in Figure 1. Depending upon whether liquid or gas is flowing through the core, different permeability values have been observed in the laboratory [6]. Liquids which do not react with the rock grain material yield equivalent liquid permeability; whereas with gases, the observed permeability value (Kg) varies linearly with reciprocal of mean pressure. At significantly high pressures Kg tends to be equivalent to the liquid permeability value. There are three classifications of permeability as absolute, effective and relative permeabilities. Absolute permeability, also called equivalent liquid permeability, is measured when the core sample is 100% saturated with a single fluid type [7,8]. When two or more fluids saturate the core, permeability of each fluid, in this case, is called effective permeability with respect to that fluid. The ratio of effective permeability of any phase to the absolute permeability of the core is called relative permeability.
Permeability is one of the fundamental petrophysical properties in formation evaluation and is usually distributed non-uniformly throughout the reservoir. It is used to describe the reservoir capacity to transmit hydrocarbons through it, frequently evaluated from well log and well test data [9]. However, in case of heterogeneous formations, well log and well test data do not provide a satisfactory value of formation permeability, so in such cases it is better to use Kozeny Carman model to characterize permeability. Generally speaking, porosity and permeability exhibit a linear relationship. However, a highly porous rock always guarantee that it will also exhibit high permeability [10]. The connectivity of pores is necessary to have a good permeability. It is very possible that a rock is highly porous, but the pores are isolated and not interconnected resulting in an impermeable rock. Pores radii distribution has a very significant effect on permeability.
Theoretical calculations show that the larger the pore radius, the larger the apparent permeability. Moreover, the apparent permeability increases with an increase in the width of pore size distribution. A permeable material has a greater number of larger, well-connected pores, whereas an impermeable material has fewer, smaller pores that are poorly connected [11-13]. Empirically, it has been observed, in most of the cases, that permeability is proportional to square of pore throat size. Therefore, the largest connected pore throats dominate the permeability of rock. A general trend of increasing permeability with porosity can be expected as shown in Figure 2, but there are various other factors such as grain shape and size, packing, compaction, pore radius, pore structure etc., which influence the relationship between porosity and permeability. Core samples with fairly low porosity and significantly high permeability exists such as Oolid grain stone formations. On the other hand, core samples with very low permeability and significantly high porosity values are also seen, such as North Sea Chalkes and California dolomites [14].
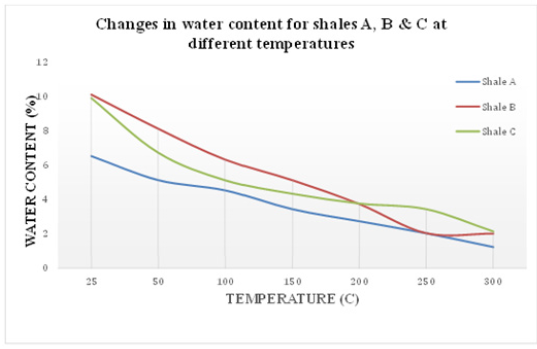
Figure 2:Changes in water content for shales A, B and C at different temperatures.
Moreover, permeability has directional characteristics depending upon the flow direction. Accordingly, the value of permeability may differ with flow direction. Porosity however is a non-directional petrophysical property of sedimentary rocks. Therefore, though correlations between porosity and permeability exist, they are strongly dependent upon pore structure [15]. Though two different core samples may have the same porosity but due to differences in their pore structure they can exhibit different permeabilities. Both pore body and pore throats, as shown in Figure 3, constitute porous volume in a core sample, but they cause different levels of permeability. It has been experimentally shown that pore body constitutes 99.31% of total porosity but determines only 0.31% of total permeability; whereas pore throats contribute 0.69% of total porosity, but controls 99.69% of total permeability (Marrow & MA, 1973). So effectively we can say that having the same porosity guarantee the same permeability as both porosity and pore structure determine permeability [16].
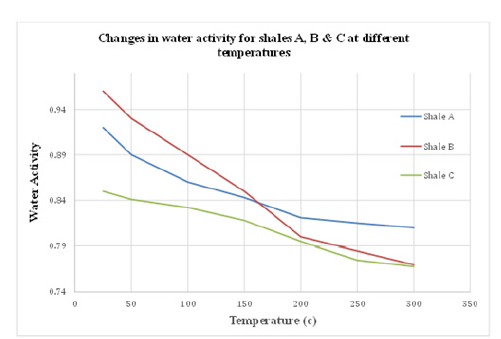
Figure 3:The impact of temperature on the water activity of shales A, B and C.
Prior wor
Wein Brandt et al. (1975) studied the temperature effects on absolute and relative permeability of sandstone samples. Dynamic displacement experiments were conducted on small cores under increasing temperature conditions [17]. Fractional flow of oil and water and Δp across the sample were noted down at various temperatures. Imbibition relative permeabilities were measured for five samples of Boise sandstone at lab temperature and 175 °F. The fluids used were water and mineral oil [18]. The effect of temperature on absolute permeability was investigated for six Boise sandstone and two Berea sandstone samples. It was observed that S_wir increased and S_or decreased with increasing temperature. Kro increased for all Sw below the ambient temperature and absolute permeability decreased with temperature increase.
Lo HY [19] studied the effect of temperature on oil-water relative permeabilities in both oil and water sands. Equipment for measuring oil and water flow rates under steady state condition was used at various temperatures, then using that data relative permeabilities of oil and water were calculated. Consolidated porous Teflon and Berea sandstone cores were used in the experiment with three oil types; Kaydol, Protol and tetradecane. Their viscosities at lab temperature were recorded as 130, 68 and 2cp, respectively. Measurements were taken at lab temperature and at 200 °F for Teflon, whereas at 300 °F for sandstone. Similar observations were made in both water-wet and oil-wet systems. With increasing temperature, relative permeability to oil was higher and residual oil saturation was lower. Also, at higher temperatures, it appeared that the rate of increase in relative permeability to water with increasing water saturation increased. Moreover, these effects were larger in systems with Kaydol and Protol than with tetradecane. It has been also established that changes in viscosity ratio(uo/uw) with temperature is a significant factor that influences relative permeability curves [20-23].
SJ Torabzadey (1984) investigated the impact of temperature and interfacial tension on consolidated and relative water/oil permeabilities. In this experimental measurements of relative permeability for high- and low-tension systems were made at a variety of temperatures between 22 °C and 175 °C. Equipment used in the experiment was conceived and built to measure the relative permeabilities of water and oil at high pressures and temperatures. The results of this study used to estimate and performance of enhanced oil recovery techniques that take into account the addition of chemicals to with hot waterfloods or as steam flood additives. Chang et al. (1999) studied the effects of temperature on capillary pressure properties of rocks. A specific equipment was designed to measure oil-brine capillary pressure of sand cores at various brine saturations for temperature ranging up to 325 °F. A refined mineral oil and 0.58% sodium chloride brine solutions were used the two sandstone and one limestone sample’s drainage and imbibition capillary pressures were tested at different brine saturations and temperatures of 175 °F, 250 °F and 300 °F, using a base pressure of 325psig [24].
The findings from the experiment confirmed when the temperature rises, there is less hysteresis between the drainage and imbibition curves and more irreducible water saturation in sandstones. Also, t was noted that during a sequence run of dropping temperatures, the irreducible water saturation at elevated temperatures appeared to be larger than it had been during the sequence runs of increasing temperatures in the prior. Though their significant changes in irreducible water saturation for limestone core with increasing temperatures. Muqeem (1995) studied the temperature influence on three-phase water-oil-gas relative permeabilities of unconsolidated sands. The measurements of flow rate and pressure drop across core samples were taken after steady state flow condition was established. Tests were conducted on Ottawa sand saturated with clean mineral oil, 1% NaCl solution and nitrogen gas. The results showed that relative permeabilities have no significant temperature dependence three-phase water relative permeability was a function of brine saturation alone. Three-phase gas relative permeability was always lower than the three phase values. Oil relative permeability seemed to vary with the saturations of the other fluids as well.
Materials and Methods
Shale properties
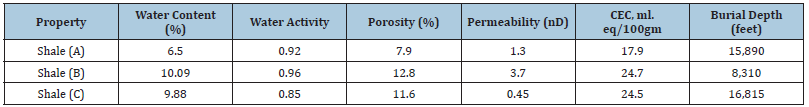
Three different shales (A, B and C) were used in this study. In order to guarantee data quality and reproducibility, several samples were carefully cut from each shale core (A, B and C) and used in this study. Proper care was taken during the handling and preparation of the samples to minimize shale exposure and interaction with the atmosphere (air). The exposure of shales to air could lead to changes in shale properties, especially the native water activity (Chenevert and Amanullah, 1997). It is important to mention that all shale samples were saturated with a simulated pore fluid (NaCl solution of 0.98 water activity). The mineralogical properties of shales A, B and C are shown in Table 1. Also, the average petrophysical properties of these shales are shown in Table 2. Detailed description of the experimental procedures used to obtain the mineralogical and petrophysical properties of shales A, B and C can be found elsewhere (Al-Bazali, 2005).
Table 1:Mineralogical properties of shales A, B and C.
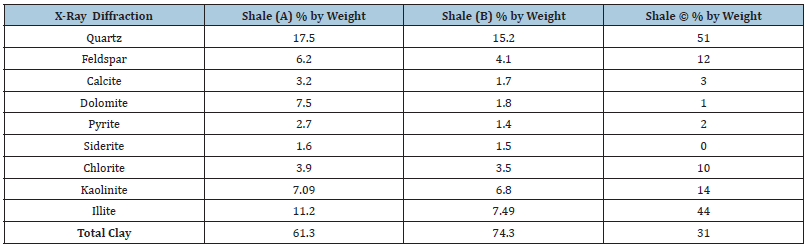
Table 2:The petrophysical & chemical properties.
Experimental procedure
Pressure transmission tests were conducted to estimate shale permeability. To measure shale permeability, osmotic flow across the shale sample needs to be eliminated; therefore, a simulated pore fluid upstream and downstream of the shale is flowed [25]. Under such conditions and in the absence of electrical and thermal gradients, the only flow that occurs will be due to a hydraulic potential gradient. Knowing the pressure differential across the shale sample as a function of time, pore fluid viscosity, shale geometry and flow rate (volume of pore fluid entering the downstream chamber), shale permeability using a transient pressure model can be calculated. A schematic of the experimental set for the pressure transmission. In this test, a shale sample is placed inside the main cell. The main cell was placed inside an oven for temperature control purposes. A simulated pore fluid was flowed across the shale sample at a constant pressure. A back pressure regulator is used to stabilize the upstream pressure at a desired value [26].
Before starting the flow across the shale, the downstream chamber is filled with a simulated pore fluid and pressurized to 50psi. It is important to mention that the initial upstream pressure is always higher than the downstream pressure so that flow occurs across the shale owing to the hydraulic potential. The pressure builds up in the downstream chamber is measured, using a pressure transducer, over time. The test is terminated 20 hours after the downstream pressure matches the upstream pressure [27]. The downstream pressure change as a function of time is recorded and used to estimate shale permeability using a pressure transient model which is explained in section 3.3. A sample of the pressure versus time graph that is used to estimate shale permeability is shown in Figure 2. Using the oven, the test temperature was changed from 25 ˚C to 300 ˚C in increments of 50 ˚C in order to investigate the impact of temperature of the permeability of shale. A detailed description of the pressure transmission test and model can be found elsewhere (AL-Bazali, 2005 and van Oort, 2003). Estimated shales A, B & C permeabilities using our developed transient model are shown in Table 3.
Table 3:Estimated shales A, B and C permeabilities used our developed transient model.
Mathematical model description
The diffusivity equation, in low permeability rocks, is used to describe our system. A full description of the mathematical model can be found in AL-Bazali TM [28].
The following procedure is adopted in order to estimate the permeability of the shale sample: i. Pressurize the downstream chamber to a desired value. ii. Allow the downstream pressure to stabilize and let this pressure be Po. The downstream pressure should equal the shale’s pore pressure. iii. Set the oven temperature at the desired value. To start with, the oven temperature was set to 25 ˚C. iv. Start the flow of the upstream fluid on top of the shale at a constant pressure (Pm). v. Record the downstream pressure build up with time (P(L,t)). vi. Plot ln [(Pm-P(L,T))/(Pm-Po)] versus time. vii. Determine the slope of the line (𝛌). viii. Calculate shale permeability as follows: k = (λμCVL / (−A)) ix. Change the oven temperature to a new value and repeat the test. In our experiment, we tested all shales (A, B & C) at 25, 50, 100, 150, 200, 250 & 300 ˚C.
Discussion
Clay sites activation
Clay swelling is a well-known phenomenon in shale interactions with salt solutions. It is approved that upon shale’s contact with water, clay swelling takes place due to water movement into shale, particularly when the shale’s chemical potential is less than the chemical potential of water (Ewy, 2017; Hale et al, 1992 & ALBazali, 2011). It has also been proposed by many researchers that heat could also produce hydrational and expansive swelling in shales (Ghassemi & Diek, 2002; Oja et al. 2015; Mirabbasi et al. 2020). The mechanism that generates swelling stresses in shal at high temperatures is still under examination [29]. I believe that high temperature could energize active swelling clays and produce a transition of inactive clays to active swelling clays. Hansen et al. (2012) argued that a clay, at room temperature, is passive and non-swelling, experiences a swelling transition as temperature increases as shown in Figure 2. The swelling of clay could narrow the passage ways between pores, also as pore throat narrowing. The narrowing of pore throats due to shale swelling, especially if the shale is confined and not allowed to expand, would eventually reduce the average pore throat radii and thus causes a reduction in shale’s permeability as per equation 4.1. The permeability of shales A and B, as depicted in Figure 1 have decreased up to a certain temperature and this could be a direct result of shale swelling promoted by thermal activation of clay sites. Inactive clay sites could thermally be transformed into active clay sites and be capable of producing clay swelling as argued above [30]. The swelling of clay occupies the available void space in pore throats and pore bodies resulting in an overall reduction of open pore space through which fluids can flow. Therefore, the permeability which measures the ability of shale to conduct fluids through it will be reduced in direct proportion to the loss of pore volume caused by shale swelling.
Pore dilation
It is claimed that pore throat size could be changed by heat through a phenomenon called pore dilation (Zhang et al. [31] and Oleas et al. 2010) as shown in Figure 3. According to equation 4.1, shale’s permeability is directly proportional to the pore throat radius squared. High temperature could have changed the mechanical structure, pore throat size and distribution of shale. Consequently, high temperature could have enhanced the size of the pore throat of shale which may have caused an increase in its permeability. It can be evidently realized from Figure 1 that the permeabilities of all shales have increased when temperature increased dramatically. This may be caused by an increase in shale pore size ‘’pore dilation’’ which could have increased shale’s permeability. It is also noticeable that the permeability of shale B has increased relatively more than that of shales A and C. This could be related to the inner shale’s fabric and mineralogical make up. According to Table 1, shale B has more clay content and less quartz and feldspar content than shales A and C which made shale B more susceptible to pore dilation than shales A and C. Unlike clay minerals, quartz and feldspar minerals are mechanically stable at high temperature and maintain their rigidity.
Water content
The amount of shale’s moisture between the grains is diminished by the application of heat. In addition, the application of heat on shale introduces air into the pores network between grains which may develop a water-air interface between the grains with a curvature radius (r) as shown in Figure 4. If the amount of moisture between grains is further reduced by heat, water will retreat deeper into the pore throats between the grains and the curvature of the air-water interface will increase, leading to a decrease in the curvature radii and a decrease in pore water pressure (Forsans & Schmitt, 1994). The reduction in curvature radii and decrease in pore water pressure in the pores network enhances the capillary entry pressure of shale which may have further complications on shale’s permeability. Figure 5 shows shales A, B & C water content (%) changes as temperature increased from 25 ˚C to 300 ˚C.
Water activity
Figure 1 shows the impact of temperature on the water activity of shales A, B & C. Initially, water activity of shales A, B & C was 0.92, 0.96 & 0.85 respectively. Figure 2 indicates a clear reduction in water activity of all shales as temperature increased from 25 ˚C to 300 ˚C. More notably, the rate of decrease in shale’s water activity was higher for temperatures between 25 ˚C to 200 ˚C. As the amount of moisture between the grains is reduced by heat, the pore fluid becomes more saturated with the ions that make up the pore fluid. In other words, more water evaporates due to high temperature leaving behind a more concentrated pore fluid since the temperature at which ions evaporate is much higher than 300 ˚C. Therefore, as the pore fluid becomes more saturated with ions and less saturated with water, the water activity of the pore fluid should drop since water activity is sensitive to ionic concentration. It is proven experimentally, that the addition of ions to solutions reduces the water activity of the solution.
Relationship between permeability, porosity and pore size
Permeability measures the connectivity between pore spaces. If the pore spaces are highly connected, this will promote permeability as it creates conductive paths for the fluid to move. Regarding shale and its mineralogical compositions, the presence of clay minerals has a great effect on permeability. If the total amount of clay increases, this means that the water activity through pore spaces will increase due to the increase in the ion concentration and directly the permeability will increase and vice versa. So, sample B shows a greater permeability with higher water activity than other samples in Table 2. As the permeability is directly proportional to radius of pore spaces and porosity, the porosity will exhibit higher values in samples with higher amount of clay with higher water activity. The shale porosity not only representing the volume of pores between the rock but also the total porosity representing the pores around and through clay particles. Sample A and C does not show porosity-permeability direct relationship. This could be due to differences at their pore throat radii and clay content.
Relationship between interfacial tension and shale permeability
Interfacial tension represents the force per unit length on the surface between two immiscible fluids. Increasing the interfacial tension will decrease the permeability as the fluid becomes more wet to the rock and resists its movement. The interfacial tension depends on the radius of pore throat, the density of the fluid and the wetting angle. Increasing the radius will decrease the interfacial tension and vice versa. As it is easy for the fluid to move through larger pore spaces than wide pore spaces. So, the resistance of movement is less in larger pore spaces. The temperature also affects the interfacial tension. Increasing the temperature will decrease the interfacial tension and in turn will increase the permeability. This is also linked to the effect of pore dilation to permeability. If the temperature increases to a higher value, the permeability exhibits higher values due to the effect of larger pore throats and reduction in interfacial tension. Shales are primarily water wet formation whereas oil, natural gases or CO2 etc. are non-wetting fluids with respect to shale. The interfacial tension between the shale pore fluid and non-wetting fluid CO2 influences the flow of CO2 through the shale samples. The flow of non-wetting fluid CO2 through the water wet shale gets difficult with Increasing Interfacial Tension (IFT), therefore we can say that permeability relative to CO2 will be reduced with increasing IFT.
Conclusion
Temperature has a great effect on the permeability of shale as it
affects the size of pore throat. Pore dilation is a key factor to measure
the turning point of permeability increase due to temperature
increase. Water activity and reactivity plays an important role in
permeability change with temperature change. A summary is
presented below:
a. The rate of decrease in shale’s water activity was higher
for temperatures between 25 ˚C to 200 ˚C.
b. As the amount of moisture between the grains is reduced
by heat, the pore fluid becomes more saturated with the ions
that make up the pore fluid.
c. Sample B shows a greater permeability with higher
water activity rather than other samples As the permeability
is directly proportional to radius of pore spaces and porosity,
the porosity will exhibit higher values in samples with higher
amount of clay with higher water activity.
d. The addition of ions to solutions reduces the water activity
of the solution.
e. Interfacial tension decreases with increasing temperature
and that can be attributed to the weakening of intermolecular
forces at the two immiscible fluids interface.
f. It is noticeable that there is clear reduction in water
activity of all shales as temperature increased from 25 ˚C to 300
˚C.
References
- Afinogenov YA (1969) How the liquid permeability of rocks is effected by pressure and temperature. SNIIGIMS 6(1): 33-42.
- Akinwunmi B, Sun L, Hirvi JT, Kasa S, Pakkanen TA (2019) Influence of temperature on the swelling pressure of bentonite clay. Chemical Physics 516: 177-181.
- Al Bazali, Zhang J, Martin EC, Sharma MM (2009) Estimating the reservoir hydrocarbon capacity through measurement of the minimum capillary entry pressure of shale caprocks. SPE Annual Technical Conference and Exhibition, USA.
- Al Bazali, Zhang J, Wolfe C, Chenevert ME, Sharma MM (2009) Wellbore instability of directional wells in laminated and naturally fractured shales. Journal of Porous Media 12(2): 119-130.
- Al Bazali (2022) On CO2 sequestration: Changes in CO2 entry pressure and adsorption capacity by heat. Journal of Porous Media 25(4): 77-93.
- Benson SM, Cole DR (2008) CO2 sequestration in deep sedimentary formations. Elements 4(5):325-331.
- Bobek JE, Mattax CC, Denekas MO (1958) Reservoir rock wettability: Its significance and evaluation. Society of Petroleum Engineers Journal 213(1): 155-160.
- Boosari SH, Atbar U, Eshkalak MO (2015) Carbon dioxide storage and sequestration in unconventional shale reservoirs. Journal of Geoscience and Environment Protection 3(1): 7-15.
- Brannan GO, Gonten WDV (1973) The effect of temperature on the formation resistivity factor of porous media. Society of Professional Well Log Analysts Annual Symposium 14.
- Bruining J, Plug WJ (2007) Capillary pressure for the sand-CO2-water system under various pressure conditions. Application to CO2 Advances in Water Resources 30(11): 2339-2353.
- Civan F (2003) Leaky tube permeability model for identification, characterization, and calibration of reservoir flow units. SPE Annual Technical Conference and Exhibition, p. 84603.
- Denekas MO, Mattax CC, Davis GT (1959) The effects of crude oil components on rock wettability. Society of Petroleum Engineers of AIME Journal 216(1): 330-333.
- Favero V, Laloui L (2018) Impact of CO2 injection on the hydro-mechanical behavior of a clay-rich caprock. International Journal of Greenhouse Gas Control 71: 133-141.
- Gonten WDV, Choudhary BK (1969) The effect of pressure and temperature on pore volume compressibility. Society of Petroleum Engineers of AIME Journal 2526.
- Katz AJ, Thompson AH (1986) Quantitative prediction of permeability in porous rocks. Phys Rev B Condens Matter 34(11): 8179-81.
- Koponen A, Kataja M, Timonen J (1997) Permeability and effective porosity of porous media. Physical Review E56: 3319-3325.
- Krevor SCM, Pini R, Benson SM (2012) Capillary pressure and heterogeneity for the CO2/water system in sandstone rocks at reservoir conditions. Advances in Water Resources 38: 48-59.
- Lo HY, Mungan N (1973) The effect of temperature on water-oil relative permeabilities in oil-wet and water-wet systems. Society of Petroleum Engineers of AIME Journal, 4505.
- Marrow NR, Ma S (1996) Relationship between porosity and permeability for porous rocks. International Symposium of The Society of Core Analysts, p. 9610.
- Mathias SA, Mc Elwaine JN, Gluyas JG (2014) Heat transport and pressure buildup during carbon dioxide injection into depleted gas reservoirs. Journal of Fluid Mechanics 756: 89-109.
- Nattavadee S, Mavko G (2017) The revised kozeny-carman equation: A practical way to improve permeability prediction in the kozeny-carman equation through pore-size distribution. SEG International Exposition, p. 6093.
- Okasha TM (2006) Investigation of the effect of temperature and pressure on interfacial tension and wettability of shu’aiba reservoir, Saudi Arabia, 3929.
- Poston SW, Ysrael S, Hossain AKMS, Montogomery EF, Ramey HJ (1970) The effect of temperature on irreducible water saturation and relative permeability of unconsolidated sands. Society of Petroleum Engineers Journal 10(2): 171-180.
- Ramey HJ, Chang SK, Marsden SS (1973) The effect of temperature on capillary pressure properties of rocks. Society of Professional Well Log Analysts Annual Symposium.
- Sarmadivaleh M, Yaseri AZA, Iglauer S (2015) Influence of temperature and pressure on quartz-water-CO2 contact angle and CO2-water interfacial tension. Journal of Colloid and Interface Science 441: 59-64.
- Sharma MM (2008) An experimental investigation on the impact of diffusion osmosis, chemical osmosis, and capillary suction on shale alteration. Journal of Porous Media 11(8).
- Sinnokrot AA, Ramey HJ, Marsden SS (1971) The effect of temperature level upon capillary pressure curves. Society of Petroleum Engineers Journal 11(1): 13-22.
- Al Bazali TM (2003) Membrane efficiency behavior in shales, The University of Texas at Austin, Austin, USA.
- Somerton WH, Selim MA (1961) Additional thermal data for porous rocks: Thermal expansion and heat of reaction. Society of Petroleum Engineers Journal 1(4): 249-253.
- Stankovich, Ewy (2000) Pore pressure change due to shale-fluid interaction: Measurements under simulated wellbore conditions. Proceedings Pacific Rocks, Fourth North American Rock Mechanics Symposium, Seattle, pp. 147-154.
- Zhang J, Chenevert ME, Bazali TA, Sharma MM (2004) A new gravimetric-swelling test for evaluating water and ion uptake of shales. SPE Annual Technical Conference and Exhibition, pp. 26-29.
© 2023 Talal Al-Bazali. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






