- Submissions

Full Text
Research & Development in Material Science
Optical Imaging for Diagnosing Cardiovascular Disease
Zhe Sun 1†, Xiumeng Hua 2†, Mengni Bao1, Han Mo1, Xiao Chen2 and Jiangping Song1,2*
1Shenzhen Key Laboratory of Cardiovascular Disease, Fuwai Hospital Chinese Academy of Medical Sciences, Shenzhen 518038, China
2State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, China
†These authors contributed equally to this work
*Corresponding author: Jiangping Song, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, China
Submission: October 26, 2022;Published: November 10, 2022

ISSN: 2576-8840 Volume 18 Issue 2
Abstract
Cardiovascular disease is one of the leading causes of death in the world population. Optical imaging has the advantages of micron-level spatial resolution, high sensitivity for subcellular localization, lack of radiation and cost-effectiveness, which make it attractive for imaging atherosclerotic plaques and clots. This article provides a brief review of optical imaging tools for cardiovascular disease diagnosis.
Keywords: Cardiovascular disease; Atherosclerosis; Optical imaging; Early diagnosis
Abbreviations: AIEgens: Aggregation-Induced Emission Luminogens; ACQ: Aggregation-Caused Quenching; CT: Microcomputed Tomography; CVD: Cardiovascular Disease; ILSI: Intravascular Laser Speckle Imaging; IVPA: Intravascular Photoacoustic Imaging; NIR: Near-Infrared; MRI: Magnetic Resonance Imaging; OCT: Optical Coherence Tomography; PAI: Photoacoustic Imaging; PET: Positron Emission Tomography
Introduction
Cardiovascular Disease (CVD) has become a major killer in the world today. Acute coronary events, including myocardial infarction, are usually triggered by rupture of unstable atherosclerotic plaques and subsequent coronary thrombosis [1]. Reducing the incidence of myocardial infarction requires identification and treatment of high-risk coronary plaques before they rupture. This unmet clinical need has driven the clinical development of multiple technologies, such as Optical Coherence Tomography (OCT) [2,3], Intravascular Laser Speckle Imaging (ILSI) [4], and Photoacoustic Imaging (PAI) [5]. These techniques have been developed to assess key plaque microstructural features such as fibrous cap thickness, plaque burden, and calcified nodules. In addition, multiple near-infrared fluorescent molecular and intravascular imaging devices have been developed to help obtain information on atherosclerotic plaques.
OCT system is a non-contact, high-resolution biological microscopic imaging equipment, which applies near-infrared light as the light source for optical interferometry and obtains biological high-resolution tissue tomography images after processing by computer system [2,3]. The axial resolution of OCT depends on the coherence length of the broadband light source, which can typically reach about 10μm, while the lateral resolution is determined by the internal focal spot size of the sample. OCT can visualize clearly the internal microstructure of vessel. Due to its imaging performance are highly consistent with those of pathological section examination, OCT is also known as “optical biopsy” in the medical field. Compared with conventional coronary angiography, OCT can show the vascular structure in three dimensions, largely overcoming the deficiency of coronary angiography in assessing the vascular profile from a two-dimensional perspective and can also accurately guide the entire process of percutaneous coronary intervention.
ILSI is a novel optical technique that provides a unique ability to quantify the viscoelasticity index of the coronary vascular system [4]. Laser speckles are granular patterns formed by the interference of coherent laser light scattered from tissues, and the resolution can reach even about 2μm. The undulation of the speckle is caused by the Brownian motion of the endogenous light scatterer. This technique has the ability to identify different mechanical features of the plaques and to perform diagnostic evaluation of unstable plaques.
PAI is an advanced hybrid imaging technique based on photogenerated ultrasound effect, combining the advantages of optical and acoustic imaging. It penetrates deeper than simple optical imaging, enabling deeper in vivo tissue imaging up to 50mm. Additionally, it has a higher resolution than conventional MRI and PET imaging, which image resolution can reach sub-micron level (0.1~1μm), realizing high-resolution molecular imaging [6]. Recent studies on the use of PAI for atherosclerosis have focused on IVPA [5], which can simultaneously detect core lipids and perivascular lipids in early atherosclerotic plaques and visualize lipid core size and its relationship with other plaque components in advanced plaques.
The development of fluorescent molecules has also played a role in advancing optical imaging. AIEgens, for example, overcome the ACQ effect of conventional fluorescent molecules and show good results as optical diagnostic reagents [7]. In particular, NIR-II emitting (900-1700nm) AIEgens exhibit reduced autofluorescence and light scattering, which facilitates imaging operations at deep lesion sites [8]. The use of nano-targeted carriers in combination with AIE molecules enables earlier detection of atherosclerotic plaques than CT and MRI [9]. These features make the AIE nanoprobe a powerful tool for monitoring atherosclerotic plaques and performing anti-AS drug screening (Figure 1).
Figure 1:(A) OCT diagnosis of vascularized coronary artery thrombosis [3]. (B) Minimally invasive devices for ILSI [4]. (C) IVPA imaging technique for visualization and diagnosis of pathological features of the arterial wall (e.g., inflammatory activity, lipid deposition) [5]. (D) High brightness AIE nano-targeting system for the diagnosis of atherosclerotic plaques [9].
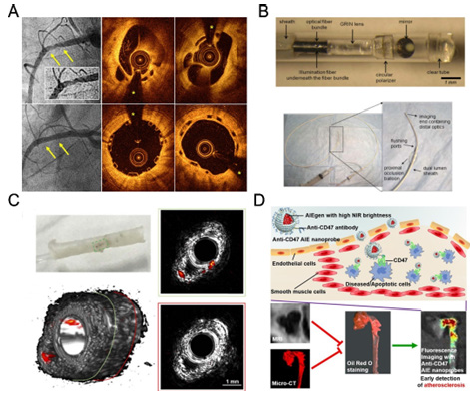
In conclusion, the construction of biomaterials and devices for optical imaging detection provides target-specific visualization tools for diagnosing cardiovascular disease, allowing for more intuitive monitoring of the disease process and providing strong support for solving clinical issues.
Acknowledgement
This research was funded by the Program for Guangdong Introducing Innovative and Entrepreneurial Teams [Grant No. 2019ZT08Y481].
References
- Zhao D, Liu J, Wang M, Zhang X, Zhou M (2019) Epidemiology of cardiovascular disease in China: current features and implications. Nature Reviews Cardiology16: 203-212.
- Aumann S, Donner S, Fischer J, Müller F (2019) Optical Coherence Tomography (OCT): Principle and Technical Realization. In: Bille JF (Edr.), High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics Cham: Springer International Publishing 2019: 59-85.
- Cuesta J, García-Guimaraes M, Bastante T, Rivero F, Alvarado T, et al. (2017) Optical coherence tomography findings in patients with recanalized coronary thrombi treated with bioresorbable vascular scaffolds. Circulation: Cardiovascular Interventions 10: e005232.
- Hajjarian Z, Toussaint JD, Guerrero JL, Nadkarni SK (2021) In-vivo mechanical characterization of coronary atherosclerotic plaques in living swine using intravascular laser speckle imaging. Biomed Opt Express 12: 2064-2078.
- Xie Z, Shu C, Yang D, Chen H, Chen C, et al. (2020) In vivo intravascular photoacoustic imaging at a high speed of 100 frames per second. Biomed Opt Express 11(11): 6721-6731.
- Hu Q, Fang Z, Ge J, Li H (2022) Nanotechnology for cardiovascular diseases. Innovation (Cambridge (Mass)) 3(2): 100214.
- Zhang Z, Kang M, Tan H, Song N, Li M, et al. (2022) The fast-growing field of photo-driven theranostics based on aggregation-induced emission. Chemical Society Reviews 51: 1983-2030.
- Li Y, Cai Z, Liu S, Zhang H, Wong STH, et al. (2020) Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nature Communications 11: 1255.
- Wang K, Gao H, Zhang Y, Yan H, Si J, et al. (2022) Highly bright AIE nanoparticles by regulating the substituent of rhodanine for precise early detection of atherosclerosis and drug screening. Advanced Materials 34: 2106994.
© 2022 Jiangping Song. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






