- Submissions

Full Text
Research & Development in Material Science
An Overview of the Reduction-Smelting Process of Iron Oxides in Modern-Day Ironmaking Technology
Joseph Ogbezode1,2*, Olusegun Ajide1, Olusoji Ofi1 and Oluleke Oluwole1
1Department of Mechanical Engineering, University of Ibadan, Nigeria
2Department of Mechanical Engineering, Edo State University Uzairue, Nigeria
*Corresponding author: Joseph Ogbezode, Department of Mechanical Engineering, University of Ibadan, Nigeria
Submission: August 10, 2022;Published: August 23, 2022

ISSN: 2576-8840 Volume 17 Issue 4
Abstract
The blast furnace and direct reduction processes have remained the hallmark of the metallic iron production route for the past decades. The challenges with the blast furnace and direct reduction route range from scarcity of metallurgical coke, high impurity, cumbersome production route, high operation cost, and environmental implications. This study gave an overview of the reduction-smelting process as a third generational alternative for the modern-day iron and steelmaking process whose hallmark is to mitigate the disadvantages that are peculiar to the convectional blast furnace and direct reduction process. This paper gave a broader research perspective to the use of a non-carbonaceous substance and less harmful reducing agents as an alternative energy source instead of the fast depleting metallurgical coke which remains the major source of carbothermal energy in the blast furnace operation. This review also substantiates the effective usage of the reduction-smelting process as a viable alternative for the iron production route in the metallurgical industry.
Keywords: Iron oxide; Blast furnace; Direct reduction; Metallurgical coke; Reduction smelting process
Introduction
The method of liquid metal production in the absence of metallurgical coke is regarded as the smelting-reduction process. This is a recent and revolutionary iron and steelmaking process used to produce hot metals which are less costly than those produced via the blast furnace route. The smelting-reduction route can also serve as an alternative to the blast furnace and direct reduction processes entail the use of two separate reactors to carry out metallurgical work on iron ore material [1]. The raw materials which could involve the combination of lump/sinter or pellet/sinter undergo a pre-reduction process inside the pre-reduction unit to obtain a metalized Direct Reduced Iron (DRI) to about 93% before it could be charged into the smelter unit stage through a counter-flow process in a melter-gasifier chamber before the separation of slags from the pure metallurgical metal takes place [2]. A slag removal process is a thermodynamic approach to separate gangue elements such as silicate, and aluminate from the reduced iron oxide metallic structure using calcium carbonate (CaCO3) or quicklime (CaO). The final product obtained is liquid pig iron or, in some cases, liquid steel. For instance, the scarcity of coking coals for blast-furnace ironmaking technology and the high cost of coke ovens are two reasons for the emergence of the reduction-smelting process as the metallurgical operation principles are close to that of a blast furnace but use non-coking coal instead of coke [3]. The reduction-smelting process consists of two major metallurgical operational stages consisting of pre-reduction and post-reduction stages (i.e. smelting-reduction units) as depicted in Figure 1; [4].
Figure 1: Schematics of iron and steel production routes by direct smelting process [4].
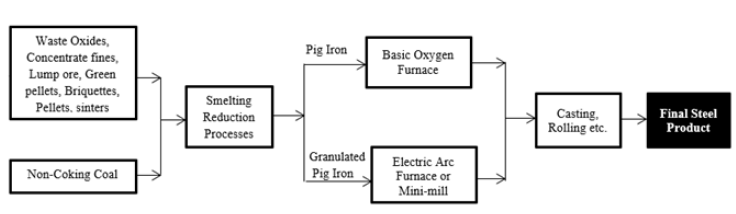
Thus, this present study gives an overview of iron production techniques in modern iron and steelmaking technology using the reducer-smelter process as a case study. The process can utilize less energy to produce tonnes of hot metals and can reduce CO2 emissions by up to 20% when compared to the typical BF route. It also can reduce metallic iron production costs by 65% compared to other iron-making conventional routes.
Discussion
In the reduction-smelting process of iron ore, the ore first undergoes a solid-state reduction with a carbonaceous material in the pre-reduction stage. The resulting product is completely reduced and then smelted in the smelting-reduction vessel where coal is gasified thus delivering heat and carbon monoxide-rich hot gas. Coal gasification takes place due to the reaction between oxygen and iron ore in a liquid state. The heat is used to smelt the iron, while the hot gas is transported to the pre-reduction unit to reduce the iron oxides. Reduced iron oxides (similar to DRI) are in turn transported to the smelting-reduction vessel for the post-reduction and smelting unit. The carbon monoxide-rich gas generated in the smelting-reduction vessel can be further oxidized to generate additional heat for smelting the pre-reduced iron oxide (Figure 2).
Figure 2: Layout of typical reduction-smelting process.
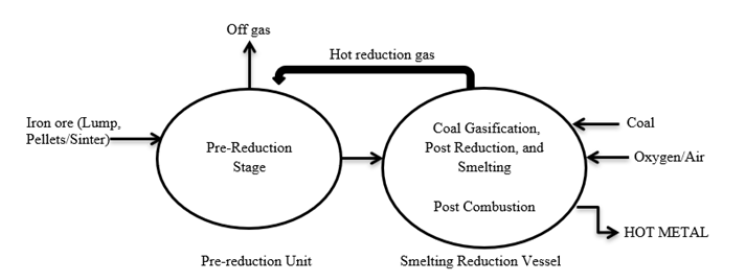
The post-combustion stages which do lead to a trade-off in the utilization of the post-combustion gas do increase prereduction potential or heat deliveries in smelted iron [5]. After post-combustion, the hot gas is transported to the pre-reduction unit and the remaining carbon monoxide is used for pre-reducing the iron oxides. The richness of carbon monoxide in the hot gas determines the degree of reduction of the DRI in the pre-reduction unit [6].
The key parameters in the smelting-reduction process are the pre-reduction, post-combustion degree, and heat transfer efficiency. The post-combustion stage shows the degree to which the CO formed in the smelting-reduction vessel by coal gasification is converted into CO2. A high degree of post-combustion results in lean gas for pre-reduction and off-gas that is quite too hot. A very low post-combustion tendency in the post-reduction chambers does result in increased CO gas and more coal consumption. The pre-reduction degree is the degree to which the iron oxides can be reduced in the pre-reduction shaft. The heat transfer efficiency is the ratio of the heat transferred from hot gases to the bath of molten iron and slag component to the heat generated by the postcombustion unit. Low heat transfer does results in off-gases rich in CO. There are many established reduction-smelting processes, though material scientists and engineers are yet to fully ascertain their qualitative tendencies and operational applicability of the process concerning their metallurgical performance [7]. Some of these processes include the COREX, Hlsmelt, Romelt, AISI Direct Steel making, Direct Iron Ore Smelting (DIOS), etc. Notable for these methods is the COREX process, recently developed in some parts of Europe. Since this is a relatively new trend in the method of iron extraction technology, there is a need to study the recent trends, importance, application, and operational and metallurgical performance of the reduction-smelting process of iron ore as a formidable approach in iron and steelmaking technology for the decade.
Conclusion and Future Perspective
Coking coals used in a blast furnace are not easy to come by, due
to material scarcity, imported duty, and high economic implications
are among the challenges militating the metallurgical industry thus
making the importation of coking coals to be quite unsustainable.
Also, the initial preparations of the iron ore (such as beneficiation,
pelletization, and concentration) before it is charged into the blast
furnace, are quite energy-consuming, time demanding, and have
high environmental and economic implications. All these processes
are not required in the reduction-smelting process. Therefore,
smelting-reduction technology remains a third-generation
approach used recently utilized to overcome the challenges
associated with the conventional blast furnace process for metallic
iron production. This study concludes and gave future research
perspectives and a more effective approach to maximize the use of
reduction-smelting technology for metallic iron production with
less environmental and economic implications to the end users
including:
a) Lower capital investment (avoiding coke ovens and
agglomeration plants and replacing the capital-intensive blast
furnaces) and the use of non-coking coal instead of expensive
metallurgical coals.
b) The technology is cost-competitive even on a relatively
small scale in that it increases operational flexibility. The
technology also shows larger flexibility in the type of raw
materials to be processed.
c) The use of hydrogen gas as a reducing agent instead of a
conventional carbonaceous substance should be encouraged.
More advances in smelting-reduction of iron oxides using
hydrogen gas as energy sources will help salvage the global
warming effects caused by the use of carbonaceous and harmful
substances in the iron and steelmaking process.
d) Finally, the reduction-smelting technology for the iron
and steelmaking process does have a clear-cut environmental
and economic advantage over the conventional blast furnace
route.
Acknowledgment
Funding: This work was supported by the Petroleum Technology
Development Fund, Nigeria
Grant Number: PTDF/ED/LSS/Ph.D./JEO/0278/19, 2019
Conflict of Interest
The authors declared they have no conflict of interest relevant to this article
References
- Ogbezode JE, Oluwole OO (2019) Reduction kinetics behavior of goethite iron ore in the CO/CO2 atmosphere from wood charcoal. International Journal of Emerging Engineering Research and Technology 7(4): 11-21.
- Li K, Wen N, Zhu M, Zheng M, Yuan L (2011) Iron extraction from Oolitic iron ore by a deep reduction process. Journal of Iron and Steel Research International 18(8): 9-13.
- Ogbezode J, Ajide O, Ofi O, Oluwole OO (2021) Determination of the reaction rate controlling resistance of goethite iron ore reduction using CO/CO2 gases from wood charcoal. Material Proceedings 3(1): 27.
- Anameric B, Kawatra SK (2008) Direct iron smelting reduction processes. Mineral Processing and Extractive Metallurgy Review: An International Journal 30(1): 1-51.
- Murakami T, Shinomiya H, Maruoka D, Kasai E (2019) Effect of types of carbonaceous material and CaO addition on reduction behavior of pre-reduced iron ore-carbon composite. ISIJ International 59(6): 1011-1017.
- Tleugabulov SM, Abikov SB, Altybaeva DK, Isupov YD, Tleugabulov BS (2015) Reductive smelting of iron ore. Steel in Translation 45(5): 351-355.
- Shi B, Zhu D, Pan J, Hu B, Wang Z (2021) Reducing process of sinter in COREX shaft furnace and influence of sinter proportion on reduction properties of composite burden. Journal of Central South University 28(3): 690-698.
© 2022 Joseph Ogbezode. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






