- Submissions

Full Text
Research & Development in Material Science
Fabrication of Ordered and Controlled MoOx 3D-Nanocubes by Anodization
Kamran Ahmad1*, AttaUllah Shah2, Muhammad Bilal1, Saira Gulfam1, Rahim Ullah2 and Zahoor Ahmad1
1Pakistan Tokamak Plasma Research Institute (PTPRI), Pakistan
2National Institute of Lasers and Optronics College (NILOP-C), Pakistan Institute of Engineering and Applied Sciences (PIEAS), Pakistan
*Corresponding author: Kamran Ahmad, Pakistan Tokamak Plasma Research Institute (PTPRI), P.O. Box 3329, Islamabad, Pakistan
Submission: July 07, 2022;Published: July 28, 2022

ISSN: 2576-8840 Volume 17 Issue 3
Abstract
This study reports the formation of uniformly distributed molybdenum oxide (MoOx) nanocubes on the Mo substrate by anodization. The electrolyte contains 1 M KOH, 0.4 M NH4F and 10:1 of Ethylene Glycol (EG) and D ionized (DI) water. The Mo samples anodized for 2, 5, 7min with graphite as counter electrode in the presence of 12V respectively. The uniformly distributed cubic morphology on the surface of molybdenum is revealed by Scanning Electron Microscope (SEM). The anodization time directly controls the size of the nanocubes, increasing anodic time directly growth the nanocubes size.
Keywords: Molybdenum oxide nanocubes; Anodization; KOH and NH4F electrolyte; Anodization time
Introduction
At microscale surface area to volume ratio is large as compared to bulk matter due to which nano/micro materials possess unique optical, magnetic, interfacial, electrical and chemical properties characteristics. The nanomaterial is very attractive in biomedicine, energy, pharmacy, food, agriculture, and environmental applications [1,2]. Recently transition metal oxides are widely investigated due to wide range of applications [3]. Molybdenum oxides (MoOx) are one of the most attractive transition metal oxides due to their special structural characteristics and low band gap (2.5-2.89eV). MoOx has two simple binary oxides; MoO3 and MoO2. MoO3 has excellent properties like low metallic electrical resistivity, high melting point, and high chemical stability. However, MoO2, MoS (converted from MoOx) has been used in many applications such as a catalyst for alkane isomerization, oxidation reactions, and as a gas sensor. It is also a promising anode material for Li-ion batteries and antibacterial activity [4-10]. Mo compounds have potential in supercapacitor and hydrogen production application [11,12].
Recently MoOx nanoparticles are fabricated by anodization in 0.02M HCl electrolytes. However, only few papers are available on the synthesis MoOx nanocubes by solid state and other methods. But still the synthesized nanocubes were dispersed very faraway and was random size and orientation of the same concept is applied here by adding NH4F to do some surface engineering by cutting edges of the particles by “F” ions [13].
In this study, MoOx nanocubes have been fabricated using the anodization technique for the first time. As “F” ion has the capability to produce pores/ nanotubes in transition metals (Ti. Ta, Fe) because it works like scissor at nano scale [14]. Also, the suitable concentration of NH4F to the electrolyte gives cohesion to the oxides film with the substrate rather it will detach the film in the electrolyte.
Materials and Methods
Anode was made of molybdenum foil of 1cm width, 2cm length and 0.1mm thickness. Pure graphite sheet (2cm width and 3cm in length and of 0.3cm thickness) has been used as cathode. Samples were washed by dipping in acetone and ethanol for 20min at 50 ℃ temperature. Then samples were cleaned by DI water for 20min at 50 ℃ and dried by air blower. Electrolyte was prepared by mixing 1M KOH, 0.4M NH4F in 20ml of EG and 2ml water stirred by magnetic stirrer for 1 hour at room temperature. The experiment was conducted for different anodization time in the presence of applied voltage of 12V, and the maximum current set was 0.8A, temperature was monitored with the help of digital thermocouple. The experimental arrangements of the anodization current are shown in the Figure 1 and the process parameters are given in Table 1.
Figure 1: Anodization current vs time for all the samples and inset photo is the setup of power supply, connecting wires, anodization bath, cooling bath and sample holder.

Table 1: Anodization parameters for the preparation of samples.
Results and Discussion
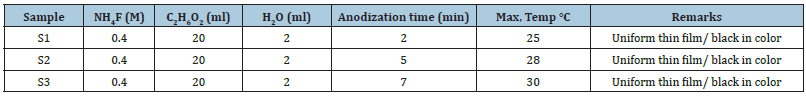
Figure 2:S1 anodized for 2min a) 50μm b) 5μm.
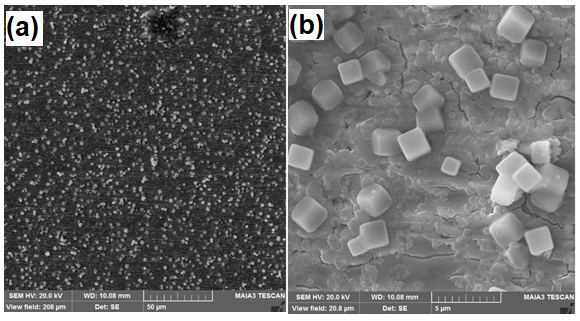
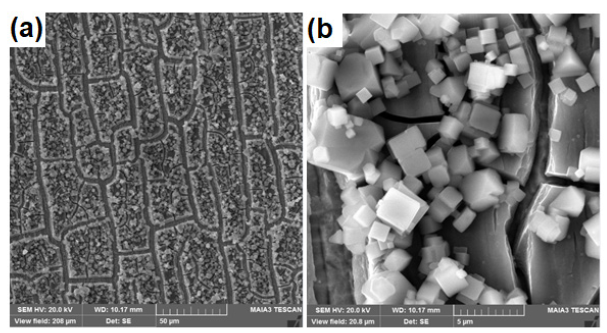
After anodization in electrolyte containing 0.4M NH4F the presence of MoOx cubes is confirmed by Scanning Electron Microscopy (SEM) as clear from the Figures 2-4. It is observed that with the increase in anodization time the formation of cubic shape particles increases and results in increase of particle size. Particle size for the sample (S1) anodized for 2min is about 1μm as can be seen in Figure 2. Particle size for sample (S2) anodized for 5min is 2μm as can be seen in the Figure 3. Similarly, particle size for sample 3 anodized for 7min is 3-4μm as shown in Figure 4. Energy Dispersive Spectroscopy (EDS) was used to measure the elemental composition of the anodized. Peaks of molybdenum, oxygen, potassium, and fluorine are shown in Figure 5. Presence of oxygen reveals the formation of MoOx cubes.
Figure 3: S2 anodized for 5min a) 50μm b) 5μm.
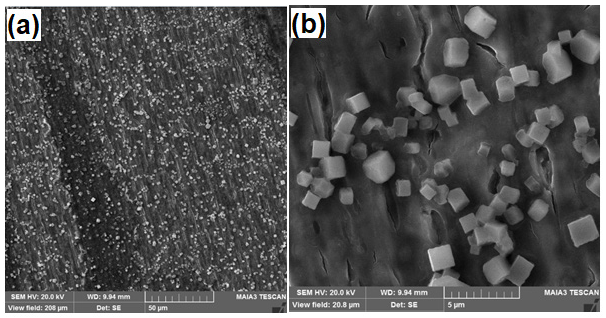
Figure 4: S3 anodized for 7min a) 50μm b) 5μm.
Conclusion
One step anodization is one of the best techniques to produce ordered MoOx cubes using NH4F, KOH in ethylene glycol and DI water as electrolyte. The anodization time controls the size of the cubes. By increasing anodization time the cubes size and its quantity increases drastically and uniformly. Anodization causes the nucleation of cubes uniformly at initial stages and for very long time it divides the surface into patches of cubes clusters.
Acknowledgment
Support from staff of NILOP and PTPRI is highly acknowledged.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kolahalam LA, Kasi Viswanath IV, Diwakar BS, Govindh B, Reddy V, et al. (2019) Review on nanomaterials: Synthesis and applications. Mater Today Proc 18: 2182-2190.
- Santhosh C, Velmurugan V, Jacob G, Jeong SK, Grace AN, et al. (2016) Role of nanomaterials in water treatment applications: A review. Chem Eng J 306: 1116-1137.
- Yanagishita Y, Masuda T, Kondo T, Masuda H (2021) Highly ordered anodic porous oxides of transition metals fabricated by anodization coambined with a pretexturing process. Electrochem Commun 123: 106916.
- Zamora-Romero N, Camacho-Lopez MA, Vilchis-Nestor AR, Castrejon-Sanchez VH, Aguilar G, et al. (2020) Synthesis of molybdenum oxide nanoparticles by nanosecond laser ablation. Mater Chem Phys 240: 122163.
- Mai L, Yang F, Zhao Y, Xu X, Xu L, et al. (2011) Molybdenum oxide nanowires: Synthesis & properties. Mater Today 14(7-8): 346-353.
- Zhan Y, Liu Y, Zu H, Guo Y, Wu S, et al. (2018) Phase-controlled synthesis of molybdenum oxide nanoparticles for surface enhanced Raman scattering and photothermal therapy. Nanoscale 10(13): 5997-6004.
- Fazio E, Spadaro S, Bonsignore M, Lavanya N, Sekar C, et al. (2018) Molybdenum oxide nanoparticles for the sensitive and selective detection of dopamine. J Electroanal Chem 814: 91-96.
- Dighore N, Jadhav S, Anandgaonker P, Gaikwad S, Rajbhoj A (2017) Molybdenum oxide nanoparticles as antimicrobial agents. J Clust Sci 28(1): 109-118.
- Sirotkin N, Khlyustova A, Titov V, Agafonov A (2020) Plasma-assisted synthesis and deposition of molybdenum oxide nanoparticles on polyethylene terephthalate for photocatalytic degradation of rhodamine B. Plasma Process Polym 17(9): 1-13.
- Muthamizh S, Suresh R, Giribabu K, Manigandan R, Praveen Kumar S, et al. (2015) Molybdenum oxide nanocubes: synthesis and characterizations. 1665(1): 1-4.
- Bowen J, Hejazi S, Chu H, Mohajernia S, Truong Nguyen N, et al. (2019) MoP-protected Mo oxide nanotube arrays for long-term stable supercapacitors. Appl Mater Today 17: 227-235.
- Yan W, Wu W, Wang K, Tang Z, Chen S (2018) Oxygen reduction reaction and hydrogen evolution reaction catalyzed by carbon-supported molybdenum-coated palladium nanocubes. Int J Hydrogen Energy 43(36): 1-10.
- Delalat F, Ranjbar M, Salamati H (2016) Blue colloidal nanoparticles of molybdenum oxide by simple anodizing method: decolorization by PdCl2 and observation of in-liquid gasochromic coloration. Sol Energy Mater Sol Cells 144: 165-172.
- Ahmad K, Bilal M, Rasheed MA, Ahmad Z, ShahA, et al. (2021) Influence of electrochemical reduction on the optical properties of TiO2 nanotubes under ambient conditions. Appl Phys A.
© 2022 Kamran Ahmad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






