- Submissions

Full Text
Research & Development in Material Science
Transition Metal Ions as Catalysts for Oxidation of Cinnamyl Alcohol - A Green Initiative
Himanshu Gupta1, Freddy Havaldar1 and D V Prabhu2*
1Department of Chemistry, St Xavier’s College, Mumbai, India
1Department of Chemistry, Wilson College, Mumbai, India
*Corresponding author: D V Prabhu, Department of Chemistry, Wilson College, Mumbai, India.
Submission: May 27, 2022;Published: June 16, 2022

ISSN: 2576-8840 Volume 17 Issue 2
Abstract
One of the 12 principles of Green Chemistry is the use of catalysts in preference to stoichiometric reagents to enhance the atom economy and yield of chemical reactions. Oxidation of alcohols to the corresponding carbonyl compounds is an industrially important reaction as it yields useful by products.
Environmentally hazardous metal ions like Cr(VI), Os(VIII) and Ru in different oxidation states have been routinely used to catalyse the oxidation of alcohols. As a green initiative, we have used the relatively inexpensive and less harmful transition metal ions to catalyse the oxidation of alcohols used in the manufacture of perfumes and fragrances.
This paper reports the kinetic study of the oxidation of the unsaturated primary perfumery alcohol, Cinnamyl alcohol by Ce(IV) in acidic medium. The oxidation was carried out under first order kinetic conditions with respect to Ce(IV) at different temperatures. From the variation of oxidation rate with temperature, the thermodynamic activation parameters of the reaction were evaluated and correlated with the proposed reaction mechanism. The effect of ionic strength on the oxidation was studied in dilute solution using Analar Grade K2SO4.
Transition metal ions Mn(II),Co(II),Ni(II),Cu(II) and Zn(II) in the concentration range [M(II)]=2.5 -5.0 x 10-4mol dm-3 were used to catalyse the oxidation of Cinnamyl alcohol by Ce(IV) in acidic medium and the sequence of their catalytic efficiencies was determined as Mn(II)>Co(II)>Ni(II)>Cu(II)>Zn(II).Mn(II) ions seem to be the best catalysts among the transition metal ions studied. Suitable reaction mechanisms have been proposed for the oxidation of Cinnamyl alcohol in the presence and absence of transition metal ion catalysts.
Keywords:Cinnamyl alcohol; Oxidation; Ce (IV); Kinetics; Transition metal ion catalysts; Catalytic efficiency; Hyper valent ions; Ionic strength; Thermodynamic activation parameters; Reaction mechanism
Introduction
Organic oxidants have been widely used for the oxidation of alcohols, but inorganic oxidants have been sparingly used [1-6]. Also, there are few reports of the kinetic studies of the oxidation of alcohols [7-10]. We have investigated the oxidation of some perfumery alcohols using organic and inorganic oxidants [11-14].
Herein, we report the transition metal ion catalysed oxidation of the perfumery primary alcohol, Cinnamyl alcohol (Molar mass 134.17g mol-1, Mol. formula C9H10O, C6H5 CH=CH CH2OH) using Ce (IV) in acidic medium. The oxidation was studied under first order kinetic conditions with respect to the inorganic oxidant i.e. [Ce(IV)] << [alc.] and the progress of the oxidation was monitored titrimetrically. The thermodynamic activation parameters were determined from the variation of oxidation rate with temperature (308-318K). K2SO4 was used to study the influence of ionic strength on the oxidation rate in dilute solution. The effects of alcohol and oxidant concentrations, ionic strength and temperature on the oxidation rate of alcohol were studied in depth.
Inexpensive and less hazardous transition metal ions, Mn(II),Co(II),Ni(II),Cu(II) and Zn(II) were used to catalyse the oxidation of Cinnamyl alcohol and the sequence of their catalytic efficiencies determined on the basis of Irving - Williams order of stability constants of the transient metal complexes formed during the oxidation process. Suitable reaction mechanisms have been suggested for the uncatalyzed and catalysed oxidation of the alcohol under investigation.
Materials and Methods
Cinnamyl alcohol was obtained from S.H. Kelkar and Co., Mumbai, India and used as received. Metal salts of required purity were procured from E. Merck, Germany. All other chemicals and reagents used were of Analar Grade.
The oxidation was studied under first order kinetic conditions with respect to the inorganic oxidant. The solutions of alcohol and oxidant in requisite amounts were allowed to equilibrate in a previously adjusted thermostat with accuracy +/- 0.1℃. When the temperature equilibrium was attained, the solutions were quickly mixed to start the reaction. Aliquots of the reaction mixture were withdrawn at regular time intervals during the course of the reaction, the reaction was quenched using ice and the unreacted oxidant was titrated against standard Ferrous ammonium sulphate in sulphuric acid medium using Ferroin as an indicator. From the linear plots of log (unreacted Ce(IV)) vs. time ,the first order rate constants (k) were determined.
The thermodynamic activation parameters were determined from the Arrhenius plots of logkvs T-1 in the temperature range 308-318K. K2SO4 was used to study the effect of ionic strength on the oxidation rate of alcohol in the range μ=0.05-0.25mol dm-3.
An identical procedure was followed to study the catalytic effect of transition metal ions on the oxidation rate of alcohol in the range [M(II)]=2.5-5.0 x 10-4mol dm-3. It was found that the oxidation rate increased with [M(II)].
Results and Discussion
Oxidation of primary alcohol, Cinnamyl alcohol by Ce(IV) in acidic medium
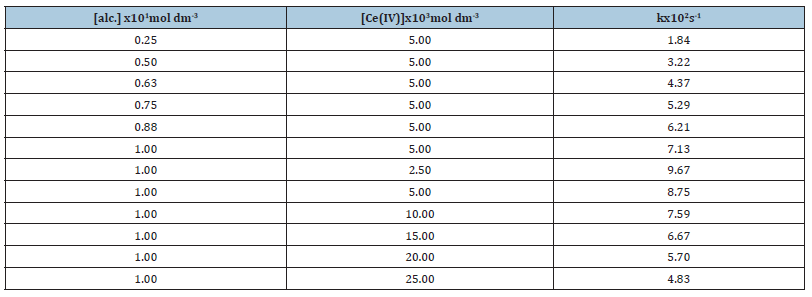
Effect of alcohol and oxidant concentrations on rate of oxidation: The rate constant data for the oxidation of Cinnamyl alcohol by Ce(IV) in acidic medium is given in Table1.The rate of oxidation increases with [alc.] but decreases with concentration of Ce(IV).
Table 1:Rate constant data for oxidation of Cinnamyl alcohol by Ce(IV) in acidic medium[alc.]=0.2mol dm-3 [Ce(IV)]=0.05mol dm-3 [H2SO4]=1.0mol dm-3 Temperature=303K
Reaction mechanism of oxidation:
k
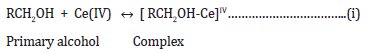
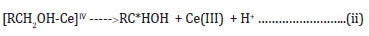
where RC*HOH is a free radical produced during the course of
the reaction.
fast
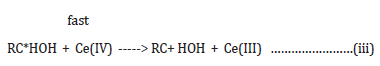
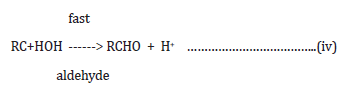
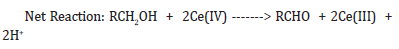
The product of the oxidation i.e. aldehyde was identified by 2,4dinitrophenylhydrazone test and later confirmed by TLC.
In aqueous acidic medium, Ce(IV) oxidizes alcohols through complexation followed by free radical generation [15-17].
The rate constant increases with [alc.] but deceases with [Ce(IV)] (Table 1).This decrease in oxidation rate is due to the formation of an unreactive dimeric [Ce(IV)]2 species which increases with [Ce(IV)][18-20] as per the reaction, 2Ce(IV) ↔ [Ce(IV)]2.
Effect of ionic strength on oxidation rate: Table 2 shows the effect of ionic strength (μ) on the oxidation rate of Cinnamyl alcohol.
Table 2:Effect of ionic strength on the oxidation rate of Cinnamyl alcohol by Ce(IV) in acidic medium [alc.]=0.1mol dm-3 [H2SO4sub>]=0.3 mol dm-3 [Ce(IV)]=5x10-3 mol dm-3 Temperature=308K
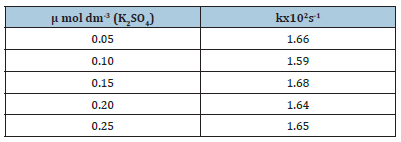
The graphs of logk vs.μ1/2 were found to be straight lines parallel to the μ1/2 axis indicating that in dilute solution, the oxidation rate is independent of ionic strength. This confirms the involvement of a non-ionic species viz. alcohol in the oxidation in accordance with the Bronsted-Bjerrum equation, log k=logk0+1.02ZAZB μ1/2 and justifies the reaction mechanism suggested for the oxidation process.
Effect of temperature on oxidation rate: The oxidation was studied at different temperatures (308-318K). The rate constants were determined from the straight-line graphs of logk vs. time. The energy of activation (E) and other thermodynamic activation parameters were determined from the Arrhenius plots of logk vs. T-1 and are recorded in Table 3.
Table 3:Thermodynamic activation parameters of the Oxidation of Cinnamyl alcohol by Ce(IV) in acidic medium [alc.]=0.1 mol dm-3 [Ce(IV)]=5x10-3mol dm-3 [H2SO4]=1.0mol dm-3
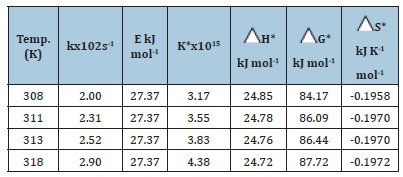
The important inferences from the thermodynamic study are:
1. The rate constant of oxidation k increases with
temperature as expected.
2. K*, the equilibrium constant for the formation of
the activated complex from the reactants increases with
temperature hence K* is a function of temperature.
3. The constant values of energy of activation E at all
temperatures indicate that the site of oxidation ie -OH bond is
the same at all temperatures.
4. The negative values of entropy of activation, ΔS* indicate
the orientation of solvent molecules around a rigid activation
complex [21]. The water molecules are tightly held to the -OH
bond which is the site of oxidation resulting in the curtailment
of the vibrational and rotational motions of the reacting system
and consequently a decrease in entropy.
Catalytic effect of transition metal ions on the oxidation rate of cinnamyl alcohol by Ce(IV) in acidic medium
Transition metal ions Mn(II),Co(II),Ni(II),Cu(II) and Zn(II) were used to catalyse the oxidation of Cinnamyl alcohol in the concentration range
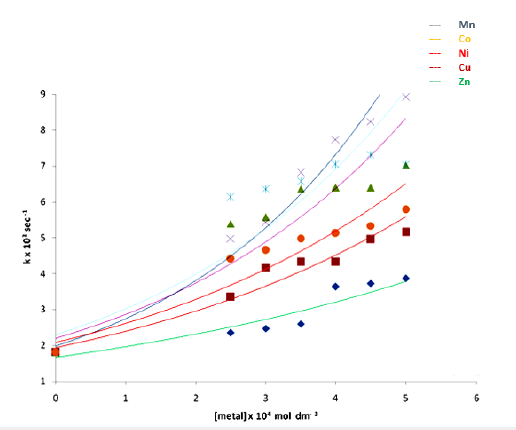
[M(II)]=2.5-5.0 x 10-4 mol dm-3(Table 4; Figure 1).
The oxidation rate of Cinnamyl alcohol increased linearly with [M(II)] and the sequence of catalytic efficiencies of the transition metal ions under study was found to be Mn(II)>Co(II)>Ni(II)>Cu(II)>Zn(II) (Table 4; Figure 1).
Table 4:Catalytic effect of transition metal ions on the oxidation rate of Cinnamyl alcohol by Ce(IV) in acidic medium [alc.]=0.1mol dm-3 [Ce (IV)]=2.5x10-3 dm-3 [H2SO4]=1.0 mol dm-3 Temperature=308K
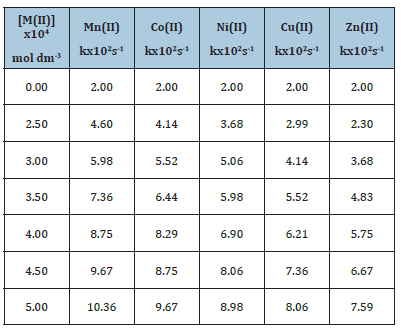
Figure 1: Catalytic effect of transition metal ions on the oxidation rate of Cinnamyl alcohol by Ce(IV) in acidic.
Bulk density: Figure 8 shows the bulk density of the various geopolymer bricks incorporated gradual ratios of ES and CCC wastes. It is obvious that the bulk density data of the prepared bricks improved and enhanced as the content of both Egg Shells (ES) and Commercial Calcium Carbonate (CCC) increased up to 12wt. % from each. This certainly attributed to the decrease of the total porosity of the samples [47,62,66]. With any further increase of either FS or CCC >12wt. %, the obtained data of the bulk density decreased. This may be due to the increase of the total porosity [66-68]. Therefore, the optimum brick batch is that containing about 12wt. % (G4) because it achieved the best results, though that the obtained data with brick batches including the egg shells were higher than those with the commercial carbonates. Hence, the higher quantities from both egg shells and/or commercial carbonates must be avoided.
Reaction mechanism of transition metal ion catalysed oxidation of cinnamyl alcohol
Mn,Co,Ni and Cu form the hypervalent ions Mn(III),Co(III),Ni(III) and Cu(III) respectively due to their low third ionization enthalpies unlike Zn which has a relatively high third ionization enthalpy (3829kJ mol-1) and its energy of solvation is not sufficient to make the 3+ state chemically stable.
In presence of Mn(II),Co(II) ,Ni(II) and Cu(II) ions:
The reaction
mechanism has been explained on the basis of the formation of an
unstable intermediate complex between hypervalent M(III) ions
and alcohol.
K
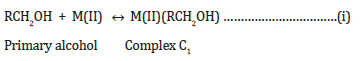
k, slow
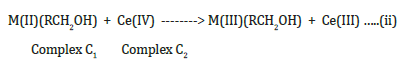
The electron transfer reaction is slow [10].
At any given time, the steady state concentrations of the intermediate complexes C1 and C2 are negligible.
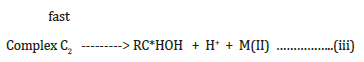
where RC*HOH is a free radical produced during the course of the reaction.
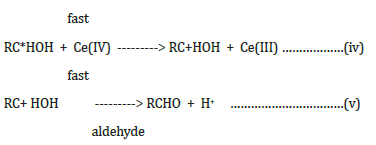
Net reaction: RCH2OH 2Ce(IV) -------> RCHO + 2H+ + 2Ce(III)
In presence of Zn(II) ions:
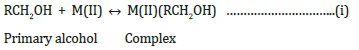
k, slow

where RC*HOH is a free radical produced during the reaction.
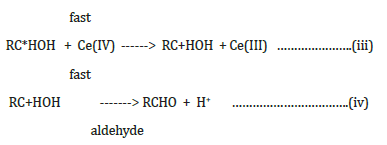
Net Reaction: RCH2OH + 2 Ce(IV) -------> RCHO + 2H+ + 2Ce(III)
Sequence of catalytic efficiencies of transition metal ions:
The catalytic efficiency of a metal ion is inversely proportional
to the stability of its complex formed as a transient short lived
intermediate during the reaction. The stability of such unstable
complexes depends mainly on the charged density of the metal ions
involved. Therefore, the stability order for the complexes of the
transition metal ions under study is expected to be
Cu(II)>Zn(II)>Ni(II)>Co(II)>Mn(II) [22,23] and the expected
sequence of catalytic efficiencies of the metal ions is
Mn(II)>Co(II)>Ni(II)>Zn(II)>Cu(II).This is a general guide
to metal ion behavior but several discrepancies have been
observed and reported in literature [24-28]. In the present
investigation, we have observed differences between the
experimentally observed sequence of catalytic efficiencies of the
transition metal ions and the theoretically expected sequence viz.
Mn(II)>Co(II)>Ni(II)>Cu(II)>Zn(II) (Table 4; Figure1).
Conclusion
Transition metal ions have been effectively used to catalyse the oxidation of Cinnamyl alcohol to Cinnamaldehyde under first order kinetic conditions with respect to the oxidant Ce(IV) in acidic medium. The reaction mechanism has been explained on the basis of the formation of intermediate complexes involving hypervalent ions, Mn(III),Co(III),Ni(III) and Cu(III). Certain discrepancies have been found between the observed sequence of catalytic efficiencies of the metal ions and the theoretically expected sequence. Among the transition metal ions studied, Mn(II) appears to be the most effective catalyst for the oxidation of Cinnamyl alcohol.
The thermodynamic activation parameters of the oxidation have been determined and correlated with the molecular dynamics of the oxidation reaction. Ionic strength has no effect on the oxidation of Cinnamyl alcohol.
References
- Hudlickly M (1990) Oxidations in organic chemistry. ACS Monographs, p.186.
- Lee SV, Madin A, Trost BM, Fleming I (Eds.), (1991) Comprehensive Organic Synthesis, Pergamon Press, Oxford, UK, 7: 251.
- Maqdziak M, Rodriquez AA, Van de Water RW, Pettur TR (2002) Org Lett 4(2): 285.
- Corey E J, Suggs JW (1975) Tetrahedron Letts 16: 2647.
- Corey E J, Boyer BL (1978) Tetrahedron Letts 19: 240.
- Corey E J, Schmidt G (1979) Tetrahedron Letts 20: 299.
- Gunasekaran S, Venkat Subramanian N (1983) Proc Indian Acad Sc (Chem Sc) 92(1): 107-112.
- Jagdeesh RV, Puttasamy (2008) J Phy Org Chem21(10): 244-258.
- Choudhary PK, Sharma PK, Banerjee KK (1979) Intl J Chem Kinetics 31: 469.
- Nandibewoor ST (1998) J Indian Chemical Society 75: 363.
- Srivastava S (2008) Asian J Chem 20: 4776.
- Das Asim K (2000) J Indian Chem Soc 77: 225.
- Prabhu D V (2007)) Kinetics and reaction mechanism of the controlled oxidation of some industrial alcohols by haloamines. J Indian Chem Soc 84: 1135-1139.
- Prabhu DV, Tandel MA, Parbat HA, Uchil Meera H (2013) A kinetic insight into the oxidation of perfumery alcohols by inorganic oxidizing agents. Research Journal of Chemistry and Environment 17(10): 69-76.
- Prabhu DV, Rana Chetana (2017) Kinetic and thermodynamic studies of the oxidation of acyclic primary perfumery alcohols using K2S2O8 and KIO4 in acidic medium. Rasayan Journal of Chemistry 10(2): 385-390.
- Parbat HA, Prabhu DV, Rana Chetana (2021) GP Globalize Research Journal of Chemistry 5(1): 72-80.
- Richardson H (1965) Oxidation in Organic Chemistry, Part 1, Wiberg KB (Ed.), Academic Press, New York, USA, p. 244.
- MinoG, Kaizerman S, Rasmussem E (1959) J Am Chem Soc 81: 1494.
- Duke FR, Forist AA (1949) J Am Chem Soc 71: 2790.
- Dorfman MK, Gryder JW (1961) Inorganic Chemistry, p.799
- Blanstin BD, Gryder JW (1975) J Am Chem Soc 79: 940.
- Shukla PS, Mehrotra RN (1973) J Inorg Nucl Chem 35: 891.
- Eichhorn GL, Trachtenberg IM (1952) J Am Chem Soc 74: 5185.
- Irving H, Williams RJP (1953) J Am Chem Soc, p. 3192.
- Mellor DP, Maley L (1947) Nature 158: 370.
(1948) Nature 161: 436.
- Williams RJP (1956) J Chem Soc.
- Prabhu DV, Parbat HA, Tandel MA (2014) Kinetic studies of the transition metal ion catalysed oxidation of some fragrance alcohols. Asian J Chemistry 26(19): 6669-6673.
- Parbat HA, Prabhu DV (2018) A kinetic approach to the oxidation of alcohols by KBrO3 in acidic medium using transition metal ion catalysts. Rasayan Journal of Chemistry 11(3): 1349-1356.
- Parbat H A, Prabhu DV, Nikalje Anna Pratima (2019) Kinetics of transition metal ion catalysed oxidation of some industrially important alcohols using Ammonium metavanadate in acidic medium. Global Journal of Science Frontier Research B-Chemistry 19(3): 35-41.
- Parbat H A, Prabhu DV (2022) Oxidation of secondary cyclic alcohols using KIO4 in acidic medium. Asian Journal of Organic and Medicinal Chemistry 7(1): 1212-1217.
© 2022 D V Prabhu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






