- Submissions

Full Text
Research & Development in Material Science
Is the Brus Equation Correct?
Pao Chi Chen*
Department of Chemical and Materials Engineering, Lunghwa University of Science and Technology, Taoyuan, Taiwan
*Corresponding author: Pao Chi Chen, Department of Chemical and Materials Engineering, Lunghwa University of Science and Technology, Taoyuan, Taiwan
Submission: May 10, 2022;Published: May 23, 2022

ISSN: 2576-8840 Volume 17 Issue 1
Short Communication
Metal oxides were widely used in the domains of materials, physics, and chemistry and have been extensively used in catalysts, fuel cells, piezoelectric materials, sensor materials, and optical components. Among various metal oxides, CeO2 is one of the extensively applied and discussed. It has a face-centered cubic structure with a high melting point (2400-2700 ℃) and possesses remarkable properties such as the number of effective redox Ce3+/Ce4+ sites and their ability to exchange oxygen [1]. Therefore, it has good optical properties, thermal stability and electrical conductivity, and oxygen storage function [2,3]. For instance, the optical property of UV-vis spectroscopy for the CeO2 nanoparticles exhibits a blue shift with respect to the bulk material due to quantum confinement, which have two limiting case, depending on the ratio of the crystallite radius to the effective Bohr radius of the electron-hole pair [4].
Therefore, the crystal size and energy gap measurements were significant in applications. Thus, a discussion of crystal size with energy gap was required. The relationship between crystal size and energy gap can be described by the Brus Equation [5]. In addition, a modified Brus equation was proposed by Ferreira et al. [4], where the modified values were smaller than that obtained from the Brus equation. In order to conduct comparisons between the Brus equation, several reported data in the literature were collected.
In general, the crystal sizes are measured by XRD with the Scherer equation or the Williamson-Hall (W-H) method [1]. On the other hand, the energy gaps are determined using absorption coefficient (α) and frequency (ν) [1], as follows
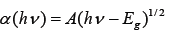
wherein A is constant, and Eg is the energy gap. Through a plot of α(hν) versus hν, the energy gap can be determined. The energy gaps of cerium oxide were found to be 3.26eV(380nm) and 3.22eV(385nm) [1], while the energy gaps for different solvents [3] were 3.44eV(361nm), 3.22eV(386nm), 3.45eV(361nm) and 3.2eV(388nm) for water, acetone, ethanol, and ethylene glycol, respectively.
The effect of size on the energy gap can be described by the Brus equation [5]:
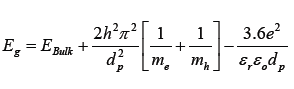
Equation (2) describes that Eg is a function of the diameter of crystal, dp. The second and third terms are represented by blue and red shifts, respectively. In addition, EBulk refers to the band gap (for CeO2; EBulk=3.15eV); me and mh are the effective masses of electron and electron hole, respectively; εr and ε0 are the relative dielectric constant of CeO2, which is 24.5, and dielectric constant at empty, respectively.
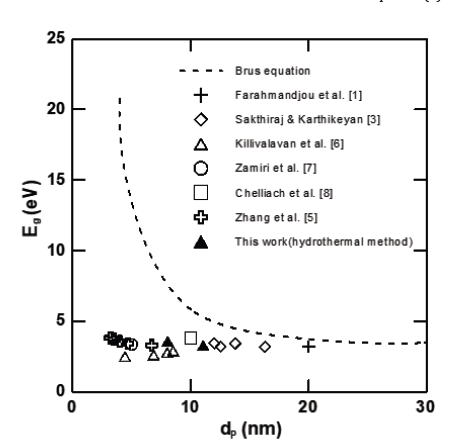
We selected me=mh=0.4m, where m is the mass of a free electron. In order to compare the reported data with the Brus equation, the equation can be calculated and expressed as a dotted line, as shown in Figure 1. In addition, the data with hydrothermal method obtained in here and from literatures with precipitation method [1,5-8] and sol-gel method [3] were also presented in this Figure 1. The particle sizes for the two runs in here were found to be 11.06nm and 8.05nm, for 100 ℃ and 150 ℃, respectively. The corresponding energy gaps were 3.3eV and 3.59eV, respectively. The data presented were used in black triangle. All data were under the dotted line, indicating that the value of Eg evaluated by the Brus equation was overestimated when dp is smaller than 20nm. According to the Brus equation, the bulk size (maximum size) can be determined by letting dEg/d(dp)=0. The final size was found to be 214nm, which is the bulk size of cerium oxide [9]. The corresponding EBulk is 3.15eV. Therefore, it needs to be modified further when we used Equation (2).
Figure 1: A plot of energy gap vs. crystal size for comparison.
References
- Farahmandjou M, Zarinkamar M, Firoozabadi TP (2016) Synthesis of cerium oxide (CeO2) nanoparticles using simple co-precipitation method. Revista Mexicana de Fisica 62: 496-499.
- Nyok M, Choonara YE, Kumar P, Kondiah PPD, Pillay V (2020) Synthesis of cerium oxide nanoparticles using various methods: Implications for biomedical applications. Nanomaterials 10(2): 242.
- Sakththiraj K, Karthikeyan B (2020) Synthesis and characterization of cerium oxide nanoparticles using different solvents for electrochemical applications. Applied Physics A 126: 52.
- Ferreira DL, Sousa JCL, Maronesi RN, Bettini J, Schiavon M A, et al. (2017) Size-dependent bandgap and particle size distribution of colloidal semiconductor nanocrystals. The Journal of Chemical Physics147(15).
- Zhang F, Jin Q, Chan SW (2004) Ceria nanoparticles: size, size distribution, and shape. Journal of Applied Physics 95: 4319-4326.
- Killivalavan G, Sathyaseelan B, Kavitha G, Baskarann I, Senthilnathan K, et al. (2020) Cobalt metal ion doped cerium oxide (Co-CeO2) nanoparticles effect enhanced photocatalytic activity. MRS Advances Materials Research Society 5: 1-13.
- Zamiri R, Ahangar HA, Kaushal A, Zakaria A, Zamiri G, et al. (2015) Dielectrical properties of CeO2 nanoparticles at different temperatures. PLoS ONE 10: e0122989.
- Chelliah M, Rayappan JB, Krishnan UM (2012) Synthesis and characterization of cerium oxide manoparticles by hydroxide mediated approach. Journal of Applied Sciences 12: 1734-1737.
- Chen PC (2022) Crystal sizes and energy gaps of cerium oxide using co-precipitation method. Materials Sciences and Applications 13: 213-231.
© 2022 Pao Chi Chen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






