- Submissions

Full Text
Research & Development in Material Science
Influence of β Phase on Corrosion Behavior of Mg-Al-Zn Alloys
Sennur CANDAN1* and Ercan CANDAN2
1Department of Biomedical Engineering, Necmettin Erbakan University, Konya, Turkey
2Department of Metallurgy and Materials Engineering, Necmettin Erbakan University, Konya, Turkey
*Corresponding author: Sennur CANDAN, Department of Biomedical Engineering, Necmettin Erbakan University, Konya, Turkey
Submission: March 30, 2022;Published: April 19, 2022

ISSN: 2576-8840 Volume 16 Issue 5
Abstract
There are numerous studies to understand the corrosion behaviors of AZ series Mg alloys and the results on the role of β phase in the corrosion of the AZ series Mg alloys are yet contradictory. In the literature, the β phase inhibits the corrosion by barrier effect or vice versa promotes corrosion by micro galvanic coupling. This review focuses on the influence of microalloying elements and the solidification rate on the β phase morphology, affecting the corrosion behavior of AZ series Mg alloys. Additionally, the oxide film-β phase relationship on the surface of the alloys also has been evaluated on their corrosion properties.
The review showed that the contradictory evaluations of the researchers about why the β phase prevents corrosion with the barrier effect or vice versa, why the β phase increases corrosion with micro galvanic coupling, attributed to variations in the production methods and related β phase morphology in microstructure.
Keywords: Mg alloy; AZ series; Intermetallic; Corrosion
Introduction
Mg-Al-Zn (AZ), Mg-Al-Mn (AM) and Mg-Al-Si (AS) series Mg alloys are used in many engineering fields from automotive industry to electronics and aerospace industry due to their high specific strength values [1-5]. As potential biodegradable implant material, it also has been attracted attention in recent years [6-11]. Mg alloys are designated by giving two letters following by two numbers. Letters presents main alloying elements (A=Al, Z=Zn, M=Mn, S=Si). Numbers indicate respective nominal compositions of main alloying elements in wt.%, e.g., AZ91 (Al ~9%, Zn ~1%).
Corrosion typically limits the use of the Mg in many applications, particularly those where it is expected to be exposed to aqueous solutions, as well as in corrosive environments from the marine atmosphere or salt on roads. Therefore, being an electrochemically highly active metal limits the use of Mg and its alloys without any protection [12,13].
There are many studies in the literature to understand the corrosion mechanisms of the AZ series Mg alloys [14-36]. However, opinions on the corrosion behavior of AZ series Mg alloys are still conflicting. Some researchers [14,15] argue that the corrosion resistance of AZ series Mg alloys noticeably improves as the amount of Al in the composition reaches to 8-9wt.%, which is due to the protective barrier effect of the Mg17Al12 (β) intermetallic phase. On the other hand, some researchers [22,23,26,30] report that the β phase does not act as a protective barrier, on the contrary, it acts as a microgalvanic cell with the alloy matrix accelerating the corrosion.
In this review, the literature with our previous and ongoing studies were compared, and the effects of production differences such as microalloying elements and solidification rate on the corrosion of AZ series Mg alloys on the β phase morphology and structure were examined. Additionally, oxide film-β phase relationship formed on the surface of the alloys were also evaluated in the frame of corrosion behaviors.
The relationship of β phase with corrosion in AZ series Mg alloys
Alloying elements and solidification rate are the main factors affecting the extend of secondary phases and its morphologies in cast metals. Therefore, parameters such as Al content, microalloying elements and their ratios, solidification rate and manufacturing methods should be considered together in the examination of corrosion behaviors of Mg alloys. In addition, the structure of the oxide film formed on the surface, related to the alloying elements, is another factor to be considered in the corrosion properties of the alloys.
β Phase
According to the Mg-Al phase diagram (Figure 1), the eutectic β phase appears to occur when Al content is above 13%. However, the eutectic β phase can also form at as low as 2wt.% Al under unstable solidification conditions during casting [26]. The morphology of the β phase is basically related to the amount of Al in the alloy [16,17,25,31,38,39], the solidification rate of the molten metal [24,32] and the addition of other alloying elements [18,21,28- 35,38,39].
Figure 1: Mg-Al equilibrium diagram [37].
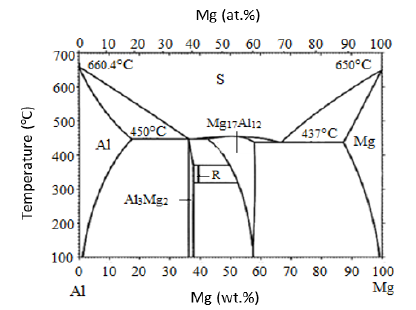
Figure 2: Morphologies of β phases in (a) AZ91 and (b) AZ91+0.5Ti alloys [21].
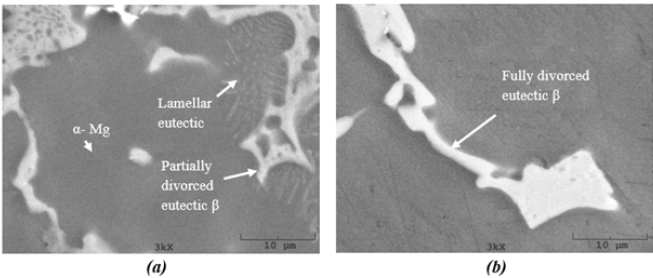
Figure 3: Corrosion in AZ91 alloy showing (a) fully eutectic β phase interrupting the corrosion progress (solidified at 8 ºC/sec) and (b) corrosion progresses via lamellar eutectic β phase (solidified at 1.4 ºC/sec). Environment: 3.5% NaCl, dark areas show corrosion products.
Fully divorced, partially divorced and/or lamellar eutectic
β-phase morphologies in Mg alloys could occur depending on
amount of Al, microalloying elements and solidification conditions
[21,25]. In Figure 2, the microstructures of AZ91 alloy and its Ti
microalloyed version are shown. Since the presence of Zn in AZ series
alloy, formation of eutectic morphology in the alloy is possible.
Due to the high segregation tendency of Zn during solidification
and the structural undercooling in front of the solid-liquid interface
in the early stages of solidification, the increase in the growth of
primary dendrites reduces the distance between the dendrites and
thus helps the formation of a fully divorced β eutectic [40,41]. The
lamellar eutectic forms in a supersaturated α-Mg solid solution juxtaposed
with the fully divorced β phase.
Predominantly lamellar and partially divorced β morphology
is formed at slow solidification and high Al content, whereas under
die casting or at low Al content fully divorced β morphology is
formed in large extend. Figure 3 shows advance of corrosion front
in AZ91 alloy having lamellar, partially divorced and fully divorced
eutectic β phases. Evidently, corrosion progresses through lamellar
and partially divorced eutectics whereas the fully eutectic β interrupts
the progress of corrosion.
Al content-β phase relationship
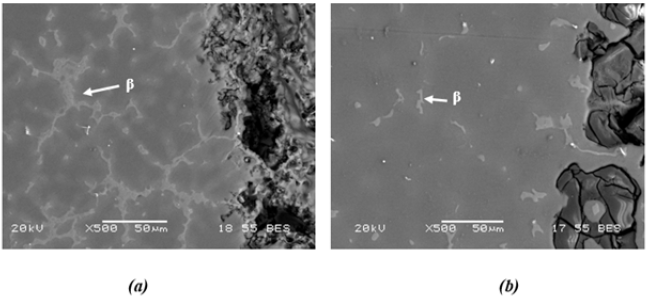
The etxtend of β phase increases with increasing Al content and transformed to a coarsened net-like structure as shown in Figure 4. It has been reported [25] that the globular shaped β phase in the alloy turned into a coarser lamellar or partially divorced β eutectic structure with increasing Al content.
Figure 4: Microstructures of (a) AZ31 alloy and (b) AZ91 alloy.

It has been reported [16,17,19] that the corrosion resistance
of AZ91 alloys was better than AZ21 and AZ31 magnesium alloys.
Pardo et al. [17], in his study on the effects of Al content in AZ31,
AZ80 and AZ91D alloys, concluded that the barrier effect of the β
phase increased with the amount of Al, and that it was the highest
in the AZ91D alloy. Wang et al. [27] observed that AZ61 alloy has
better corrosion resistance when compared to AZ31 alloy in their
study. Researchers [16,17,19,27] attributed the higher corrosion
resistance of AZ91 alloy to two key factors; the enrichment of Al
in the matrix and the presence of β phases that acting as a protective
barrier during corrosion. Contrary to the above researchers
[16,17,19,27], some researchers [22,23,26,30] reported that
β-phases increase corrosion by forming microgalvanic cells with
the alloy matrix. Studies on the corrosion behavior of the above
mentioned [17,19,22,23,27] AZ series Mg alloys have not been
comprehensively evaluated. For example, in references [17,27], the
AZ31 and AZ61 alloys are compared with the AZ91 alloy and discussed
only by considering the Al content of the alloys, regardless
of their production method. In the evaluated studies [17,27], AZ91
alloy is in billet form and AZ31 alloy is in rolled plate form, and it
is inevitable that their microstructures will be different. However,
it is known that alloying elements [18,21,25,28-35], solidification
rate [20,24,32] and production methods [33] significantly affect the
microstructure and therefore have serious effects on the corrosion
behavior of the alloys.
Candan and Candan [25] conducted systematic comparative
studies on the corrosion behavior of AZ21, AZ41, AZ61 and AZ91
Mg alloys (with similar solidification rate and impurity content).
Their results showed that the corrosion resistance was weaker in
studies with Al addition above 4wt.%. The reduction in corrosion
resistance of the alloys was attributed to the presence of lamellar
and partially divorced β eutectics promoted by high Al content.
Therefore, these results showed that β caused micro galvanic corrosion
rather than a barrier effect.
Microalloying
Corrosion properties of AZ series Mg alloys can be improved
by microalloying with Y, Ce, Sb, Bi, Si, Ca, Ho, Sr, Sc, Pb or Ti
[5,18,21,24,28,29,32,34,35,38,42-65].
Zhang et al. [45] reported that 0.8% Y addition has a significant
effect on the improvement of corrosion resistance of AZ91 alloy due to its grain refining effect. Adding Y element to AZ series alloys
formed the Al2Y phase. At the same time, it not only reduced the
extent of β phase with its grain refiner feature, but also encouraged
the formation of more homogeneously distributed β in the matrix.
As a result, Y addition (up to 0.8%) has a positive effect on the corrosion
resistance of AZ91 alloys. On the other hand, an increase
in the corrosion rate of AZ91E alloy containing high amount of Y
(2%Y) has been reported [46]. Witte et al. [5], compared in vivo
corrosion behavior of WE43 and LAE442 (containing rare earth elements)
Mg alloys with AZ31 and AZ91 Mg alloys. As a result of this
study, it was stated that the corrosion resistance was highest in the
LAE442 alloy, while the AZ31, AZ91 and WE43 alloys dissolved at
similar rates. In the studies carried out to improve corrosion resistance
of the AZ91 alloys [43,63,64], the effect of Ce addition was
investigated. The addition of Ce to the AZ91D alloy resulted in a
net-like β-phase that acted as a corrosion barrier [63]. In a study
on the effect of Ho on AZ91D alloys [35], it was reported that the
corrosion resistance of the alloy improved. Limited research on Ho
to date [35] suggested that the addition of 0.2% and 0.4% Ho to the
AZ91D alloy could improve the corrosion resistance by reducing
extent of the β phase due to the formation of Al and Ho-containing
intermetallic phases. It was reported [52] that the addition of Sc in
AZ91 alloys refined the microstructure with the formation of Al3Sc
phases, which suppressed the formation of the β phase. In the comparison
between AZ91E and AZ91E alloy containing 0.1% Sc [46],
it was reported that an increase in corrosion resistance occurred in
alloys with Sc added.
Addition of Sb or Bi to AZ91 alloy caused the formation of Mg3Sb2
or Mg3Bi2 phases [48]. Therefore, these phases, which are the cathode
relative to the matrix, reduced the corrosion resistance of the
alloy [48]. It has been reported [48] that the addition of Sb and Bi
to the alloy together caused a significant decrease in the corrosion
resistance of the alloy. Addition of Bi to Mg-Al alloy, needle-shaped
Mg3Bi2 phase had been formed even if the Bi concentrations were
below the solubility limit [61]. In another studies [46,62] on the
effect of adding Bi to AZ91 alloy, presence of Mg3Bi2 in the structure
decreased the corrosion resistance. In a study, carried out by
Srinivasan et al. [42], it was observed that the addition of Si and
Sb together on the corrosion behavior of AZ91 Mg alloy increased
the corrosion resistance. They attributed this to the formation of
thin polygonal shaped Mg2Si phases and being more effective in
preventing corrosion than the Chinese script shaped Mg2Si phases.
It has been reported [47,49] that addition of Ca to the AZ91 Mg
alloy increased the corrosion resistance of the alloy due to the mesh
Al2Ca phase that acted as an effective barrier against corrosion. Wu
et al. [34] in their study, effects of Ca and RE elements on the corrosion
behavior, microstructure and mechanical properties of AZ91
were investigated. The addition of Ca has increased the corrosion
resistance compared to the addition of RE. The increase in corrosion
resistance of AZ91 alloy with 1% Ca added was based on the
formation of Al2Ca phase. Although these studies [34,47,49] support
each other, in general, when Ca additions are close to or above
the solubility limit (1.35%), acceleration in the corrosion rate has
also been reported [65-67].
When Sr is added above the solubility limit (>0.1wt.%) in AZ
series alloys, it increased grain size, binary eutectic Al4Sr, Mg17Sr2
and Mg2Sr structures [68]. These phases reduced the β phase ratio
and homogenized its distribution which increased the corrosion resistance
of the alloy [54,68,69].
Pb microalloying changed the microstructure of AZ series cast
Mg alloys and caused a more homogeneous β phase distribution
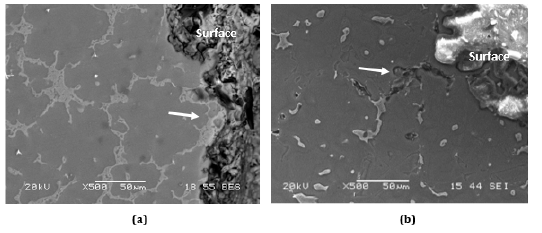
[18,70]. In our previous studies [18] on mechanical and corrosion
behaviors of AZ91 Mg alloys containing Pb (0.2-1.0 wt.%), the corrosion
resistance of the alloy increased significantly with the addition
of trace amounts of Pb. The results showed that the addition of
Pb suppressed the formation of the β phase (Figure 5), resulting in
better corrosion resistance and mechanical properties in the AZ91
alloy [18].
Figure 5: Cross-sectional SEM images of (a) AZ91 and (b) AZ91+0.5Pb Mg alloys after corrosion (Dark areas show corrosion products).
In our other studies [21,24,71] on the microalloying of AZ91
and AZ31alloys with Ti, it was observed that the corrosion resistances
of the alloys containing Ti were significantly improved. The
effects of Ti were attributed to; (1) changes in the morphology and
distribution of the β phase (transformation of β phase from lamellar
and partially divorced eutectic to fully divorced eutectic (Figure
2), and (2) increased amount of Al in α-solid solution. In another
study [72] on the corrosion resistance of AZ91 alloys containing
0.1-0.5wt.% Ti and Cr, it was reported that the corrosion resistance
increased with increasing Ti and Cr addition. Studies by Ai and
Quan [55] on the effect of Ti on the corrosion properties of AZ91
Mg alloys showed that the addition of 0.1-0.5wt.% Ti improves the
corrosion resistance of the cast Mg alloy in agreement with Refs
[21,24,71,72]. The effect of trace amount of Ti microalloying to
AZ31, AZ61 and AZ91 Mg alloy on microstructure and corrosion
behavior was studied by another research group also [38,57,58].
In the first study by Choi and Kim [58], the effects of Ti addition
(0.01-0.02%) on microstructure and corrosion properties of AZ61
Mg alloys were investigated and the results showed that Ti addition
changed morphology and grain size. They argued that 0.01%
Ti alloying significantly increased corrosion resistance, but 0.02%
Ti addition reduced corrosion resistance and Ti addition had no
systematic effect.
In other studies, by Choi and Kim [38,57], the effects of the addition
of trace amounts of Ti to AZ91 and AZ31 alloys on the corrosion
behaviors of the alloys were investigated. In the results, it
was observed that the addition of Ti refined the β phase in both
alloys and the corrosion resistance of the alloys. The results of the
research conducted by this group show that the corrosion resistance
of AZ31, AZ61 and AZ91 alloys containing Ti is much better
than alloys without Ti. These results support our previous works
[21,24,71].
The above studies reveal that improving the corrosion resistance
of AZ series Mg alloys can be achieved by microalloying
elements that promote formation of fully divorced β phase in the
matrice.
Oxide film
In a dry atmosphere and at room temperature, Mg reacts rapidly to form Mg oxide (MgO) and shows good corrosion resistance in these environments due to the MgO film.
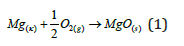
In the aqueous medium, a semi-passive Mg(OH)2 oxide film forms on the surface of Mg and its alloys. When this porous and completely non-protective Mg(OH)2 oxide film layer is left in environments containing Cl- ions for a long time, the oxide film deteriorates over time as stated in the following reaction (2).
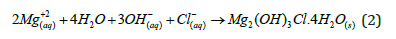
The resulting corrosion products (Mg2(OH)3Cl.4H2O) form a protective layer on the Mg surface by preventing oxygen and other corrosion environments, resulting a decrease in the corrosion rate [73].
Figure 6: Surface morphology showing the disruption of continuity of the oxide film on AZ91 alloys by coarse β phase immersed in 3.5% NaCl solution for 1/4h [25].
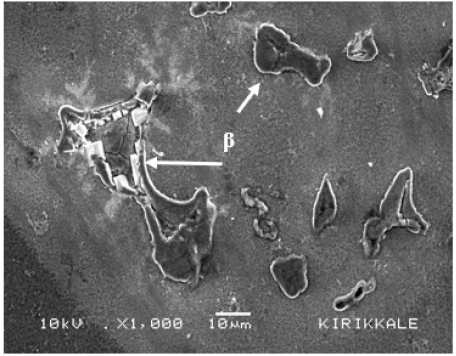
Studies [30,36,53,73-77] have been carried out in various environments on the oxidation behavior of Mg-Al alloys. Alloying Mg with Al changes the microstructure of matrice and the structure of the oxide film on the surface, increasing its resistance to the degrading effect of aggressive Cl- ions. Song and Atrens [36] reported that the AZ91 alloy film layer consists of three layers: the inner layer (rich in Al2O3), the middle layer (predominantly MgO), and the outer layer (Mg(OH)2). In a more recent study, Esmaily et al. [74] showed that the Al enrichment was evident in the inner layer of the film on the AZ91 alloy and that Al was present in the oxidized form. Both Song and Atrens [36] and Esmaily et al. [74] suggested that the positive effect of Al on the corrosion resistance of the Mg alloy was due to the protective properties of the Al-enriched layer inside the film. i.e. the formation of the Al2O3 layer in the interior could act as a passive film between surface of the alloy and the semi-passive film. However, in a study [25] on the corrosion behavior of AZ series Mg alloys (AZ21, AZ41, AZ61 and AZ91), it was observed that the corrosion resistance was lower in alloys with Al content above 4%. With the addition of Al, the globular shaped β phase in the AZ41 alloy has transformed into a coarser lamellar or partially divorced β eutectic net-like structure in the AZ91 alloy (Figure 4). Although AZ61 and AZ91 alloys contain more Al than AZ41 alloy, the reason for lower corrosion resistance could be based on the morphology and amount of the β phase and the interruption of the continuity of the oxide film by coarse β phase on the alloy surface. The interruption of the continuity of the oxide film on the alloy surface is shown in Figure 6, resulting from the coarsened intermetallic structure. MgO hydration occurs when exposed to water. Hydration of MgO has converted cubic MgO to hexagonal Mg(OH)2, which has twice the volume of the oxide, resulting in significant degradation of the film and formation of unstable regions [53].
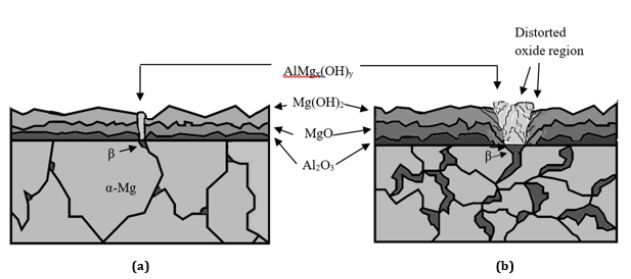
On the other hand, the growth rate of the oxide film formed on the β phase, such as AlMgx(OH)y, has occurred faster than that of the oxide film formed on the α-Mg [53]. Thus, volume changes between two oxide films (Mg(OH)2 and AlMgx(OH)y) could disrupt the interface between α-Mg and β phase. This phenomenon is shown schematically in Figure 7. The pressure created by the effect of the growth differences between the different oxide films could cause ruptures and the continuity of corrosion with long-term contact with the metal surface directly. Samaniego et al. [22] stated that in contrast to the beneficial effect of Al, Mg alloys containing a coarser β phase may corrode faster than alloys containing low Al if the protective effect of the pre-existing surface oxide film is disrupted.
Figure 7: Schematic representation of oxide films (a) AZ21 and AZ41, (b) AZ61 and AZ91 [25].
Solidification rate
Under faster solidification conditions, networking structure of β phase has suppressed and transformed into a finer, more separated and isolated phases in AZ91 alloy as shown in Figure 8; [24].
Figure 8: Microstructure of AZ91 alloy solidified at (a) 1.4 ºC/sec (b) 8 ºC/sec [24].

In some studies [17,26,27,34,35,42,44] given above sections on the corrosion behavior of the AZ series Mg alloys, it is inevitable that compositions and solidification conditions of the samples could differ since the samples were probably obtained from different suppli ers. For example, AZ31 and AZ61 alloys were compared with AZ91 alloy and discussions were made considering the Al content of the alloys ignoring the production route of the samples [17,27]. In the studies [17,27], AZ91 alloy was in the billet form whereas AZ31 alloy was in the rolled plate form. As mentioned above alloying elements [5,18,21,24,28-32,42-65], solidification rate [20,24,32,48] and production methods [33] significantly alter the microstructure which in turn affect the corrosion resistance of the alloy. The effect of solidification rate on AZ91 Mg alloys on corrosion has reported by Tanverdi [32]. As the solidification rate increased, the grain size of the AZ91 alloy has decreased, the amount of Al differed in the grain and grain boundaries, which segregated more at the grain boundaries. Therefore, the corrosion resistance of AZ91 alloy increased with increasing solidification rate [75-77].
Celik [78], Candan et al. [24] reported that the higher solidification rate could improve the corrosion resistance since it causes the grain size of the alloy to become finer. In a study [33], cast AZ91D Mg alloy has presented better corrosion resistance than its ingot form. Since AZ91D alloy is used in the pressure die casting processes, samples solidify much faster than its ingot form that probably promoted the formation of fully eutectic β phase supporting the argument. Celik [78], Candan et al. [24] have investigated the effect of solidification rate on microstructure and corrosion properties of AZ91 Mg alloys microalloyed with 0.5wt.% Pb or Ti. The results showed that the corrosion resistance of the alloy increased as the solidification rate increased. The higher corrosion resistance of the AZ91 alloy containing Pb or Ti has attributed to the smaller size of the β-phase network structure in the microstructure with the increase in solidification rate, as well as the suppression of β-phase formation by Pb and Ti.
Conclusion
In this review, the effects of microalloying elements and solidification
rate on the β-phase structure on the corrosion of AZ series
Mg alloys were examined by comparing the literature data with our
previous and ongoing studies. At the same time, the corrosion properties
of the alloys, the β phase structure and the oxide film-β phase
relationship formed on the surface were also evaluated.
1) The review reveals that improving the corrosion behavior of
AZ series Mg alloys could be achieved with modification of the
β-phase by microalloying. Especially, Ti microalloying seems to
be more promising.
2) The corrosion resistance of AZ series Mg alloys could be improved
by increasing the solidification rate that resulted in a
finer grain structure and formation of fully divorced β phase.
3) Volume changes between two different oxide films, (Mg(OH)2
and AlMgx(OH)y), formed on the surface of the alloys during
corrosion could disrupt the interface between α-Mg and
β-phase. The pressure created by the effect of the growth differences
between the different oxide films could cause ruptures
and the progression of corrosion with long-term contact
to the metal surface.
4) Conflicting evaluations on the corrosion of AZ series Mg alloys
may result from the production methods of the alloys used and
the differences in the β phase morphology accordingly.
5) The β phase could play a role as an inhibitor or an enhancer
of corrosion, depending on its structure and morphology. If
the β phase network structure in the microstructure is in the
form of fully divorced and narrow meshed net-like structure,
the progression of corrosion may be blocked vice versa it may
promote corrosion by micro galvanic coupling when it is in the
lamellar morphology.
References
- Friedrich HE, Mordike BL (2006) Magnesium technology: Metallurgy, design data, applications. Baskı, Springer-Verlag Berlin Heidelberg, Germany, p. 677.
- Luo AA, Sachdev AK (2012) 12-Applications of magnesium alloys in automotive engineering. Advances in Wrought Magnesium Alloys, Woodhead Publishing, UK, pp. 393-426.
- Pekgüleryüz MÖ, Kainer KU, Kaya AA (2013) Fundamentals of magnesium alloy metallurgy. In: Mihriban Pekguleryuz, Karl Kainer, Aslan Kaya(Eds.), (1st edn), Woodhead Publishing, Pennsylvania.
- Manuel MV, Singh A, Alderman M, Neelameggham NR (2015) Magnesium Technology 2015. Wiley Publishing, USA, pp. 301-347.
- Blawert, Hort N, Kainer KU (2004) Automotive applications of magnesium and its alloys. Transactions of the Indian of Metals, pp. 397-408.
- Witte F, Kaese V, Haferkamp H, Switzer E, Meyer Lindenberg A, et al. (2005) In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 26(17): 3557-3563.
- Staiger MP, Pietak AM, Huadmai J, Dias G (2006) Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 27(9): 1728-1734.
- Poinern GEJ, Brundavanam S, Fawcett D (2012) Biomedical magnesium alloys: a review of material properties, surface modifications and potential as a biogradable orthopaedic implant. AJBE 2(6): 218-240.
- Waizy H, Seitz JM, Reifenrath J, Andreas W, Friedrich WB, et al. (2013) Biodegradable magnesium implants for orthopedic applications. AJBE 48(1): 39-50.
- Agarwal S, Curtin J, Duffy B, Jaiswal S (2016) Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Materials Science and Engineering 68: 948-963.
- Gerengi H, Kaya E, Cabrini M (2017) The potential of pure magnesium to be used as a biodegradable material. J Advanced Technologies in Turkish 6: 9-25.
- Gray JE, Luan B (2002) Protective coatings on magnesium and its alloys-a critical review. Journal of Alloys and Compounds 336(1-2): 88-113.
- Hornberger H, Virtanen S, Boccaccini AR (2012) Boccaccini AR Biomedical coatings on magnesium alloys-A review. Acta Biomaterialia 8(7): 2442-2455.
- Hehmann F, Froes FH, Young W (1987) Rapid solidification of aluminium, magnesium and titanium. Journal of Metals 39(8): 14-21.
- Lunder O, Lein JE, Aune TK, Nisancioglu K (1989) The role of magnesium aluminuim (Mg17Al12) phase in the corrosion of magnesium alloy AZ91. Journal Corrosion 45(9): 741-748.
- Anık M, Avci P, Tanverdi A, Çelikyürek I, Baksan B, et al. (2006) Effect of the eutectic phase mixture on the anodic behavior of alloy AZ91. Materials and Design 27(5): 347-355.
- Pardo A, Merino MC, Coy AE, Viejo F, Arrabal R, et al. (2008) Influence of microstructure and composition on the corrosion behaviour of Mg/Al alloys in chloride media. Electrochimica Acta 53(27): 7890-7902.
- Candan S, Unal M, Turkmen M, Koc E, Turen Y, et al. (2009) Improvement of mechanical and corrosion properties of magnesium alloy by lead addition. Materials Science & Engineering A 501(1-2): 115-118.
- Salman SA, Ichinove R, Okido M (2010) A Comparative electrochemical study of AZ31 and AZ91magnesium alloy. International Journal of Corrosion.
- Wang J, Huang S, Guo S, Wei Y, Pan F (2013) Effects of cooling rate on microstructure, mechanical and corrosion properties of Mg-Zn-Ca alloy. Transactions of Nonferrous Metals Society of China 23(7): 1930-1935.
- Candan S, Unal M, Koc E, Turen Y, Candan E (2011) Effect of titanium additions on mechanical and corrosion behaviours of AZ91 magnesium alloy. Journal of Alloys and Compounds 509(5): 1958-1963.
- Samaniego A, Llorente I, Feliu S Jr (2013) Combined effect of composition and surface condition on corrosion behaviour of magnesium alloys AZ31 and AZ61. Corrosion Science 68: 66-71.
- Singh IB, Singh M, Das S (2015) A comparative corrosion behavior of Mg, AZ31 and AZ91 alloys in 3.5% NaCl solution. Journal of Magnesium and Alloys 3(2): 142-148.
- Candan S, Celik M, Candan E (2016) Effectiveness of Ti-micro alloying in relation to cooling rate on corrosion of AZ91 Mg Alloy. Journal of Alloys and Compounds 672: 197-203.
- Candan S, Candan E (2018) A Comparative study on corrosion of Mg-Al-Zn alloys. Transactions of Nonferrous Metals Society of China 28(4): 642-650.
- Cheng YL, Qin TW, Wang HM, Zhang Z (2009) Comparison of corrosion behaviors of AZ31, AZ91, AM60 and ZK60 magnesium. Trans Nonferrous Met Soc China 19(3): 517-524.
- Wang L, Shinohara T, Zhang BP (2012) Electrochemical behaviour of AZ61 magnesium alloy in dilute NaCl solutions. Materials and Design 33: 345-349.
- Guangyin Y, Yangshan S, Wenjiang D (2000) Effects of Sb addition on the microstructure and mechanical properties of AZ91 magnesium alloy. Scripta Materialia 43(11): 1009-1013.
- Yu F, Guohua W, Chunquan Z (2006) Influence of cerium on the microstructure, mechanical properties and corrosion resistance of magnesium alloys. Materials Science and Engineering: A 433(1-2): 208-215.
- Song G, Atrens A, Wu X, Bo Zhang (1998) Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride. Corrosion Science 40(10): 1769-1791.
- Koç E (2013) Investigation of the mechanical behavior of biodegradable magnesium alloys due to corrosion. Turkey.
- Tanverdi A (2005) The effect of solidification rate and Si and Y additives on the corrosion behavior of magnesium alloy AZ91. Turkey.
- Song G, Atrens A, Dargusch M (1999) Influence of microstructure on the corrosion of die cast AZ91D. Corrosion Science 41(2): 249-273.
- Wu G, Fan Y, Gao H, Zhai C, Zhu YP (2005) The effect of Ca and rare earth elements on the microstructure, mechanical properties and corrosion behaviour of AZ91D. Materials Science and Engineering: A 408(1-2): 255-263.
- Zhou X, Huang Y, Wei Z, Chen Q, Gan F (2006) Improvement of corrosion resistance of AZ91D magnesium alloy by holmium addition. Corrosion Science 48(12): 4223-4233.
- Song G, Atrens A (1999) Corrosion mechanisms of magnesium alloys. Advanced Engineering Materials 1(1): 11-33.
- Baker H (1998) Alloy Phase Diagrams. ASM Handbook, (3rd edn), USA.
- Choi HY, Kim WJ (2017) The improvement of corrosion resistance of AZ91 magnesium alloy through development of dense and tight network structure of Al-rich α phase by addition of a trace amount of Ti. Journal of Alloys and Compounds 696: 736-745.
- Koc E, Mathan BK, Unal M, Candan E (2015) Influence of zinc on the microstructure, mechanical properties and in vitro corrosion behavior of magnesium-zinc binary alloys. Journal of Alloys and Compounds 648: 291-296.
- Nave MD, Dahle AK, St John DH (2000) The role of zinc in the eutectic solidification of magnesium‐aluminium‐zinc alloys. Magnesium Technology 2000, (1st edn), Wiley Publishing, USA, pp. 243-250.
- Dahle AK, Lee YC, Nave MD, Schaffer PL, St John DH (2001) Development of the as-cast microstructure in magnesium-aluminium alloys. Journal of Light Metals 1(1): 61-72.
- Srinivasan A, Ningshen S, Mudali UK, Pillai UTS, Pai BC (2007) Influence of Si and Sb additions on the corrosion behavior of AZ91 magnesium alloy. Intermetallics 15(12): 1511-1517.
- Liu W, Cao F, Zhong L, Zheng L, Jia B, et al. (2009) Influence of rare earth element Ce and La addition on corrosion behavior of AZ91 magnesium alloy. Materials and Corrosion 60(10): 795-803.
- Wenwen D, Yangshan S, Xuegang M, Feng X, Min Z, et al. (2003) Microstructure and mechanical properties of Mg–Al based alloy with calcium and rare earth additions. Materials Science and Engineering A 356(1-2): 1-7.
- Zhang J, Niua X, Qiua X, Liu K, Nan C, et al. (2009) Effect of yttrium-rich metal on the microstructures, mechanical properties and corrosion behavior of die cast AZ91 alloy. Journal of Alloys and Compounds 471(1-2): 322-330.
- Südholz AD, Birbilis N, Bettles CJ, Gibson MA (2009) Corrosion behavior of Mg alloy AZ91E with atypical alloying additions. Journal of Alloys and Compounds 471(1-2): 109-115.
- Wang Q, Chen W, Zeng X, Lu Y, Ding W, et al. (2001) Effect of Ca addition on the microstructure and mechanical properties of AZ91 magnesium alloy. Journal of Materials Science 36(12): 3035-3040.
- Guangyin Y, Yangshan S, Wenjiang D (2001) Effects of bismuth and antimony additions on the microstructure and mechanical properties of AZ91 magnesium alloy. Materials Science and Engineering: A 308(1-2): 38-44.
- Hirai K, Somekawa H, Takigawa Y, Higashi K (2005) Effects of Ca and Sr addition on mechanical properties of a cast AZ91 magnesium alloy at room and elevated temperature. Materials Science and Engineering A 403(1-2): 276-280.
- Guohua W, Yu F, Hongtao G, Chunquan ZZ, Ping Y (2005) The effect of Ca and rare earth additions on the microstructure, mechanical properties and corrosion behavior of AZ91D. Materials Science & Engineering A 408(1-2): 255-263.
- Yu F, Guohua W, Hongtao G, Guanqun L, Chunquan Z (2006) Influence of lanthanum on the microstructure, mechanical property and corrosion resistance of magnesium alloy. Journal of Materials Science 41(17): 5409-5416.
- Yao SJ, Yi DQ, Yang S, Cang XH, Li WX (2007) Effect of Sc on microstructures and corrosion properties of AZ91. Materials Science Forum 546-549: 139-142.
- Liu M, Zanna S, Ardelean H, Frateur I, Schmutz P, et al. (2009) A first quantitative XPS study of the surface films formed, by exposure to water, on Mg and on the Mg-Al intermetallics: Al3Mg2 and Mg17Al12. Corrosion Science 51(5): 1115-1127.
- Kim WC, Nam ND, Kim JG, Lee JI (2011) Effect of strontium on corrosion properties of AZ91 magnesium alloy. Electrochemical and Solid-State Letters 14(11): C21-C24.
- Ai X, Quan G (2012) Effect of Ti on the mechanical properties and corrosion of cast AZ91 magnesium alloy. The Open Materials Science Journal 6: 6-13.
- Boby A, Srinivasan A, Pillai UTS, Pai BC (2015) Mechanical characterization and corrosion behavior of newly designed Sn and Y added AZ91 alloy. Materials and Design 88: 871-879.
- Choi HY, Kim WJ (2016) Development of the highly corrosion resistant AZ31 magnesium alloy by the addition of a trace amount of Ti. Journal of Alloys and Compounds 664: 25-37.
- Choi HY, Kim WJ (2014) Significant effects of adding trace amounts of Ti on the microstructure and corrosion properties of Mg–6Al–1Zn magnesium alloy. Journal of Alloys and Compounds 614: 49-55.
- Nordlien JH, Nişancıoglu K, Ono S, Masuko N (1997) Morphology and structure of water-formed oxides on ternary MgAl alloys. Journal of the Electrochemical Society 144(2): 461-466.
- Südholz AD (2010) On the development of magnesium alloys with improved corrosion resistance. PhD Thesis, Materials Engineering, Monash University, Melbourne, VIS, Australia.
- Li QA, Zhang Q, Li CQ, Wang YG (2011) Effects of Bi on mechanical properties of magnesium alloy AZ81. Advanced Materials Research 284-286: 1693-1696.
- Zhou W, Aung NN, Sun Y (2009) Effect of antimony, bismuth and calcium addition on corrosion and electrochemical behaviour of AZ91 magnesium alloy. Corrosion Science 51(2): 403-408.
- Fan Y, Wu G, Zhai C (2006) Influence of cerium on the microstructure, mechanical properties and corrosion resistance of magnesium alloy. Materials Science & Engineering A 433(1-2): 208-215.
- Mert F, Blawert C, Kainer KU, Hort N (2012) Influence of cerium additions on the corrosion behaviour of high pressure die cast AM50 alloy. Corrosion Science 65: 145-151.
- Bornapour M, Celikin M, Cerruti M, Pekgüleryüz M (2014) Magnesium implant alloy with low levels of strontium and calcium: the third element effect and phase selection improve bio-corrosion resistance and mechanical performance. Materials Science and Engineering: C 35: 279-282.
- Leil TA, Hort N, Dietzel W, Blawert C, Huang Y, et al. (2009) Microstructure and corrosion behaviour of Mg-Sn-Ca alloys after extrusion. Transactions of Nonferrous Metals Society of China 19(1): 40-44.
- Tamura Y, Sugimoto Y, Soda H, McLean A (2013) Structure and mechanical properties of Mg-Ca and Mg-Ca-Zr alloys. Journal of The Japan Institute of Light Metals 63(8): 279-285.
- Zhao P, Wang Q, Zhai C, Zhu Y (2007) Effects of strontium and titanium on the microstructure, tensile properties and creep behavior of AM50 alloy. Materials Science and Engineering A 444(1-2): 318-326.
- Nam ND, Kim WC, Kim JG, Shin KS, Jung HC (2011) Corrosion resistance of Mg-5Al-xSr alloys. Journal of Alloys and Compounds 509(14): 4839-4847.
- Zhao MC, Deng YL, Zhang XM (2008) Strengthening and improvement of ductility without loss of corrosion performance in a magnesium alloy by homogenizing annealing. Scripta Materialia 58: 560-563.
- Candan S, Emir S, Candan E (2022) In vitro degradation behavior of Ti-microalloyed AZ31 magnesium alloy in simulated body fluid. Journal of Materials Engineering and Performance 31: 1-10.
- Ünver G (2011) Investigation of the effect of the addition of Ti and Cr alloy elements to AZ91 magnesium alloy on the properties. M.Sc. Thesis, in Turkish, Sakarya University, Sakarya, Turkey.
- Lin C, Li X (2006) Role of CO2 in the initial stage of atmospheric corrosion of AZ91 magnesium alloy in the presence of NaCl. Rare Metals 25(2): 190-196.
- Esmaily M, Blucher DB, Svensson JE, Halvarsson M, Johansson LG (2016) New insights into the corrosion of magnesium alloys -the role of aluminum. Scripta Materialia 115: 91-95.
- Shih TS, Liu JB, Wei PS (2007) Oxide films on magnesium and magnesium alloys. Materials Chemistry and Physics 104: 497-504.
- Feliu SJr, Galván JC, Pardo A, Merino MC, Arrabal R (2010) Native air-formed oxide film and its effect on magnesium alloys corrosion. The Open Corrosion Journal 3: 80-91.
- Czerwinski F (2012) Oxidation characteristics of magnesium alloys. JOM 64(12): 1477-1483.
- Çelik M (2014) Investigation of the effect of cooling rates on mechanical and corrosion properties of AZ91 magnesium alloys. MSc Thesis, in Turkish, Bilecik Şeyh Edebali University, Bilecik, Turkey.
© 2022 Sennur CANDAN. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






