- Submissions

Full Text
Research & Development in Material Science
Controlled Drug Release from Loratadine-Loaded Pectin Film Dosage Forms
Yoshifumi Murata*, Sae Sugimoto, Chieko Maida and Kyoko Kofuji
Faculty of Pharmaceutical Science, Hokuriku University, Japan
*Corresponding author: Yoshifumi Murata, Faculty of Pharmaceutical Science, Hokuriku University, Ho-3, Kanagawa-machi, Kanazawa 920-1181, Japan
Submission: February 21, 2022;Published: February 25, 2022

ISSN: 2576-8840 Volume 16 Issue 4
Abstract
Film Dosage forms [FD] prepared using water-soluble polysaccharides swell and transform into a gelatinous substance in an aqueous medium. In this study, we prepared FDs using pectin as the film base and modified them using additives such as chitosan. We then incorporated loratadine, an anti-allergic agent, into the FDs and investigated the film disintegration and drug dissolution profiles in a limited volume of aqueous medium. The FD swelled when it came in contact with physiological saline, leading to disintegration. The film disintegration rate decreased with increasing pectin concentration. However, the drug dissolution rate was not affected by the disintegration profile. The addition of chitosan to the film base decreased the drug dissolution rate, although the disintegration rate of the FD scarcely changed. Modifying the FD by adding chitin to the film base did not influence the drug dissolution rate. When the FD was soaked in artificial gastric juice, loratadine immediately dissolved. A similar drug release profile was observed when chitosan was added to the film base solution. FDs prepared with pectin may be an attractive dosage form, particularly for patients who find it difficult to swallow conventional oral dosage forms.
Keywords: Film dosage form; Pectin; Chitosan; Loratadine; Controlled drug release
Introduction
Film dosage forms [FDs] prepared with water-soluble polymers rapidly swell and disintegrate in bodily fluids and release the drug incorporated into the polymer matrix [1-3]. FDs can be used to deliver drugs to local disease sites. Oral disintegration films have been noted for treating localized conditions in the oral cavity, when the drug incorporated in the FD is soluble in saliva [4-7]. We have previously reported FDs loaded with miconazole, an antifungal agent, that has been approved as a therapeutic agent for oral candida [8]. The miconazole FD prepared with a natural polysaccharide, sodium alginate, as a film base, swelled on contact with an aqueous medium and released miconazole on disintegration.
Recently, trials have been conducted to develop a dosage form for administering drugs to patients with dysphagia [9-11]. The forms are useful for administering drugs to people with difficulty swallowing, such as the elderly [9,12]. For example, oral disintegrating tablets disintegrate immediately after administration with little water; it is then transformed into a suspension that can be easily swallowed [13]. Jelly-like formulations are also useful for orally administering medicines [14-17]. Since an FD swells, disintegrates, and transforms into a gelatinous substance in a limited volume of aqueous medium, it can be used to administer medicines to a person with difficulty swallowing, provided the drug dissolution can be controlled in the oral cavity [6,18].
Pectin is a water-soluble polysaccharide that used as a gelling agent in the food industry. Polysaccharides are used for preparing oral dosage forms as they are deemed safe for consumption; thin films of polysaccharides can be formulated using simple methods, without dissolving them in an organic solvent or using heat [19,20]. Previously, we investigated the characteristics of FDs prepared with various pectins and found that FDs disintegrated upon contact with limited volume of fluid [21]. If the drug dissolution rate can be controlled to achieve efficient delivery to the absorption site, FDs formulated with pectin can be a novel dosage form used to administer drugs to patients with difficulty swallowing. In the present study, we modified FDs formulated using commercially available pectin as the film base using additives such as chitosan. We then loaded the FDs with loratadine [LRD], an anti-allergic agent, and investigated the disintegration and drug dissolution profile in a limited volume of aqueous medium.
Experimental
Materials
Two species of pectin [GENU pectin, Sansho Co. Ltd., Osaka, Japan] were used as the film base, and some polysaccharides were used as additives, as shown in Table 1. LRD was purchased from Combi-Blocks Ltd. [San Diego, CA, USA]. Hydroxylamine was purchased from Wako Pure Chemical Industries Ltd. [Osaka, Japan]. Water-soluble carbodiimide and 1-cyclohexyl-3-(2- morpholinoethyl)carbodiimide metho-p-toluenesulfonate [CMEC] were purchased from Aldrich Chemical Co. [Milwaukee, WI]. All other chemicals were of reagent grade and obtained from commercial sources. The reagents used for the pectin colorimetric assay were as follows: 20mM hydroxylamine in ion-exchanged water and 0.1 M CMEC in 2% pyridine-HCl buffer [pH 5.0].
Table 1: Film bases and additives for formulating FDs.
FD Formulation
The FD was prepared as follows: 2-4% [w/w] pectin or the solution containing 0.5% additive, such as chitosan was dispersed in deionized water to prepare the film base solution. LRD [10mg] was added to 10g of the base solution and then the mixture was thoroughly mixed by sonication and poured [3g each] into individual plastic petri dishes [diameter: 54mm]. The dishes were maintained at 40 °C for 24h. The circular films were then transferred to a desiccator. The thickness of each film was measured at 10 points using a micrometer [CLM1-15QM; Mitutoyo, Kawasaki, Japan] with a set pressure of 0.5N. Three films were measured, and the mean thickness was calculated.
Film disintegration test
One of the films was placed in a plastic dish; 10mL of physiological saline preheated to 37 °C was poured. The dish was shaken at 300rpm in an incubator [SI-300; As One Co., Osaka, Japan] at 37 °C. Aliquots [0.3mL] were withdrawn at regular intervals using a plastic syringe [Terumo Co., Tokyo, Japan] and filtered through a syringe-driven filter unit [Millex-HV, pore size: 0.45mm, Millipore Co., Danvers, MA, USA]. An equal volume [0.3mL] of physiological saline was added to the dish in the incubator at 37 °C to maintain a constant volume. Aliquots [0.1mL] of the filtered solution were combined with 0.9mL of ion-exchanged water in test tubes and vortexed. The amount of pectin in each sample solution was measured using the method described below [22]. liquots [1mL] of hydroxylamine and CMEC were added to 1mL of the sample solution, vortexed, and incubated at 40 °C for 20min; 20mM FeCl3 in 0.1M HCl [3mL] was added to the mixture. The absorbance of the solution in a quartz cell [1-cm path length] was measured at 480nm using a spectrophotometer [UV-1200; Shimadzu, Kyoto, Japan]. The absorbance was normalized to that of the blank reagent. For each test, a calibration curve was constructed using a fresh set of pectin standard. Each test was performed in triplicates.
Drug dissolution test
The sample solution was prepared using the method described earlier in section 2.3. Next, 80-μL aliquots of the filtered sample solution were placed in microtest tubes [1.5mL], and 720μL of methanol was added to precipitate the polysaccharide. The samples were mixed and centrifuged [7,700 × g, 5min; H-1300; Kokusan Co., Saitama, Japan]. The supernatant was then injected into the highperformance liquid chromatography [HPLC] stationary column.
The HPLC system [Hitachi Co., Tokyo, Japan] consisted of a pump [L- 2130], UV detector [L-2400], autosampler [L-2200], and chromateintegrator [D-2500] connected to a packed column [150mm × 4.6mm, Cosmosil 5C18-MS-II, Nacalai Tesque, Inc., Kyoto, Japan]. LRD concentration was determined at ambient temperature using a mobile phase consisting of 10mM triethylamine-phosphate buffer [pH 7.0] and methanol [13:37], at a flow rate of 1.0mL/min [23]. The detector wavelength was set at 244nm. The drug dissolution test was also performed in 10mL of JP XVIII 1st medium [pH 1.2]. Each test was performed in triplicates.
Solubility of LRD
The solubility of the LRD was measured in 10mL of ionexchanged water containing 15mg of additives. LRD was added to the test solution and the mixture was shaken at 37 °C for 24h. The suspension was withdrawn using a plastic syringe, preheated to 37 °C, and filtered using a syringe-driven filter unit [pore size: 0.45μm]. The solution was diluted with methanol, mixed, and centrifuged [7,700 × g] for 5min. The supernatant was injected into the HPLC column.
Results and Discussion
When 2-5% pectin containing LRD was cast, a thin circular film was obtained after the solvent evaporated from the base solution, as shown in Figure 1. An approximately 70μm-thick film was formed using 4% PT-L or PT-H. FDs were also obtained using a base solution containing an additive, such as chitin or chitosan.
Figure 1: Images of FDs prepared with pectin and loaded with LRDs. [a] 2% PT-L; [b] 2% PT-H; [c] 4% PT-H; [d] 2% PT-L + 0.5% Chitin; [e] 2% PT-L + 0.5% CS-F; [f] 4% PT-H + 0.5% β-CS.
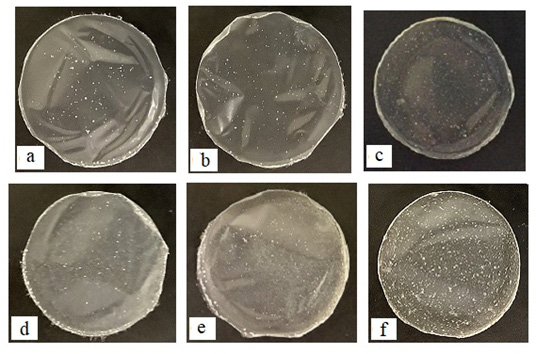
Although the disintegration profile of an FD is crucial for characterizing them, assessing the disintegration of FD merely by observation with the naked eye is challenging. In this study, the disintegration profiles of the films were assessed by measuring the amount of pectin dissolved in the test medium from each FD. The FD prepared using a water-soluble material swelled and disintegrated on coming in contact with aqueous media. For the FD prepared with 2% PT-L, 60% of the pectin dissolved in the test solution after 10min, as shown in Figure 2. The film disintegration rate decreased with increasing PT-L concentration. For example, only 27% pectin dissolved after 10min from the FD prepared with 4% PT-L. In both FDs prepared with 2% PT-H and 4% PT-H, the dissolution ratios of pectin from FDs at 10min were 38% and 20%, respectively.
Figure 2: Dissolution profiles of pectin from FDs prepared with 2-4% pectin.
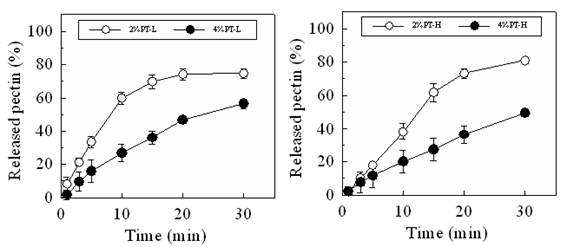
Figure 3: Dissolution profiles of LRD from FDs prepared with 2-4% pectin in the physiological saline.
LRD gradually dissolved from the FD, as the FD swelled and disintegrated in the test medium. Figure 3 shows the drug dissolution profiles of the FDs prepared with 2% or 4% pectin. The amount of LRD dissolved from the FD prepared with 2% PT-L at 10min was 0.39±0.01mg and that from the FD prepared with 4% PT-L at 10min was 0.35±0.08 mg. Similar drug dissolution rates were obtained when the FDs were prepared using PT-H.
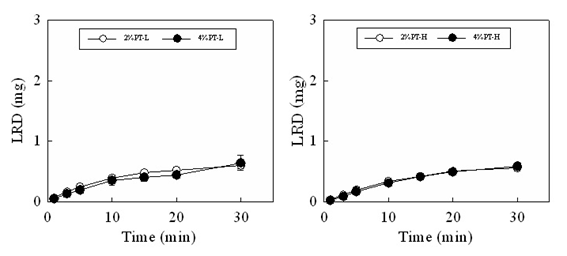
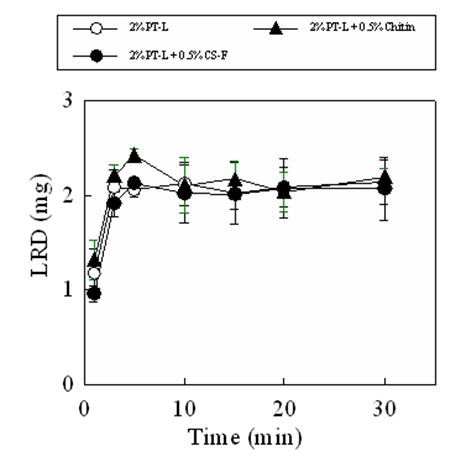
Figure 4 shows the disintegration profiles and drug dissolution profiles of the FDs modified by additives. The disintegration rate of FD scarcely changed on adding either CS-F or chitin to the film base. For the FD prepared with 2% PT-L and 0.5% chitin or 0.5% CS-F approximately 50% of the incorporated pectin dissolved within 10min. In contrast, the drug release rate from the FD decreased when chitosan was utilized as an additive. In FDs prepared with 2% PT-L and 0.5% CS-F, 0.06±0.01mg of the drug dissolved within 10min. Adding 0.5% chitin to the film base did not affect the drug dissolution rate. The suppression of the drug dissolution rate by adding chitosan to the film base solution was also observed in FDs prepared with 2% PT-H or 4% PT-L, as shown in Table 2. These results showed that the decrease in the drug dissolution rate after adding chitosan is not due to the suppression of film disintegration in physiological saline. When CS-B, CS-H, CS-C or β-CS was added to the base solution, the LRD dissolution rate decreased, as shown in Table 3. In all cases, the amount of LRD that dissolved from the FD prepared with 4% PT-H and 0.5% chitosan at 10min was below 0.12mg (equivalent to approximately 4% of the amount of LRD incorporated in the FD). This result shows that LRD did not dissolve from the FDs modified by chitosan in physiological saline.
Figure 4: Dissolution profiles of pectin and LRD from FDs prepared with 2% PT-L containing additive in the physiological saline.
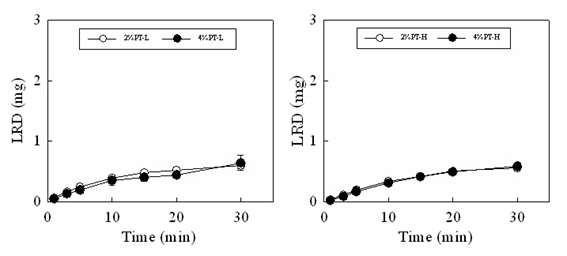
Table 2: Effect of additives on LRD dissolution rate in physiological saline.
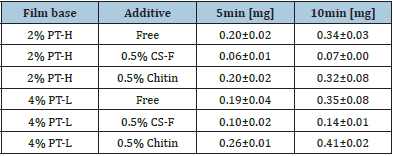
Table 3: Effect of additives on LRD dissolution rate in physiological saline from FD prepared with 4% PT-H.
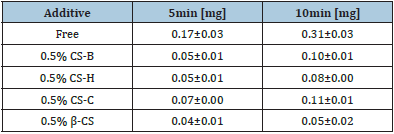
Table 4 shows the aqueous solubility of LRD at 37 °C. The aqueous solubility of LRD increased on adding chitin. The addition of chitosan to the solution barely affected the aqueous solubility of LRD. Therefore, the decrease in the drug dissolution rate of FDs modified with chitosan was not due to reduced aqueous solubility.
Table 4: Solubility of LRD in water containing 0.15% additive at 37 °C.
LRD is administered orally and absorbed from the gastrointestinal tract; therefore, LRD loaded in the FDs is expected to dissolve in the stomach. Figure 5 shows the drug dissolution profiles of FDs in artificial gastric juice. When the FD prepared with 2% PT-L was soaked in the acidic medium, it immediately released LRD; more than 2mg of the drug dissolved at 5min. A similar drug dissolution profile was observed when either chitin or CS-F was added to the film base solution. Based on these results, it can be understood that on oral administration, LRD from the FD prepared with pectin immediately dissolves in the stomach, regardless of the presence or absence of additives, such as chitosan.
Figure 5: Dissolution profiles of LRD from FDs prepared with pectin in artificial gastric juice.
Conclusion
FD is a unique dosage form that swells and disintegrates in limited volumes of medium. In this study, we formulated FDs using two types of pectin and an additive as the film base, loaded with a model drug, LRD. We then investigated the disintegration profile of the FD and the drug dissolution profile. Although FDs immediately swelled and disintegrated in physiological saline and LRD gradually dissolved from the form, the dissolution rate of LRD could be controlled by adding chitosan to the film base. FDs prepared with pectin and containing an active compound may be an attractive dosage form, particularly for patients who have difficulty swallowing conventional oral dosage forms.
Conflict of Interest
The authors declare no conflicts of interest regarding the publication of this manuscript.
References
- Strickley RG (2019) Pediatric oral formulations: An updated review of commercially available pediatric oral formulations since 2007. J Pharm Sci 108(4): 1335-1365.
- Gennari CGM, Selmin F, Minghetti P, Cilurzo F (2019) Medicated foams and film forming dosage forms as tools to improve the thermodynamic activity of drugs to be administered through the skin. Curr Drug Deliv 16(5): 461-471.
- Mohamad SA, Salem H, Yassin HA, Mansour HF (2020) Bucco-adhesive film as a pediatric proper dosage form for systemic delivery of propranolol hydrochloride: In-vitro and in-vivo Drug Des Devel Ther 14: 4277-4289.
- Ferreira AO, Brandão MAF, Raposo FJ, Polonini HC, Raposo NRB (2017) Orodispersible films for compounding pharmacies. Int J Pharm Compd 21(6): 454-461.
- Gupta MS, Kumar TP (2020) Characterization of orodispersible films: An overview of methods and introduction to a new disintegration test apparatus using LDR - LED sensors. J Pharm Sci 109(10): 2925-2942.
- Visser JC, Wibier L, Mekhaeil M, Woerdenbag HJ, Taxis K (2020) Orodispersible films as a personalized dosage form for nursing home residents, an exploratory study. Int J Clin Pharm 42(2): 436-444.
- Khalid GM, Selmin F, Musazzi UM, Gennari CGM, Minghetti P, et al. (2021) Trends in the characterization methods of orodispersible films. Curr Drug Deliv 18(7): 935-946.
- Murata Y, Kanemaru H, Tsushima M, Maida C, Kofuji K (2018) Film dosage forms prepared with alginate for oral candidiasis treatment. Res Dev Material Sci 4(3): 1-5.
- Lau E TL, Steadman KJ, Cichero JAY, Nissen LM (2018) Dosage form modification and oral drug delivery in older people. Adv Drug Deliv Rev 135: 75-84.
- Taylor S, Glass BD (2018) Altering dosage forms for older adults. Aust Prescr 41(6): 191-193.
- Lee HS, Lee JJ, Kim MG, Kim KT, Cho CW, et al. (2020) Sprinkle formulations-A review of commercially available products. Asian J Pharm Sci 15(3): 292-310.
- Drumond N, Stegemann S (2020) Better medicines for older patients: Considerations between patient characteristics and solid oral dosage form designs to improve swallowing experience. Pharmaceutics 13[1]: 32.
- Sharma S, Singh K (2020) Oral disintegrating tablets - An updated patent perspective. Recent Pat Drug Deliv Formul 14(3): 166-190.
- Kaae JK, Spejlborg ML, Spork U, Bjørndal K, Eriksen JG (2020) Reducing late dysphagia for head and neck cancer survivors with oral gel: A feasibility study. Dysphagia 35(2): 231-241.
- Patel S, Scott N, Patel K, Mohylyuk V, McAuley WJ (2020) Easy to swallow "Instant" jelly formulations for sustained release gliclazide delivery. J Pharm Sci 109(8): 2474-2484.
- Aziz ZHA, Katas H, Omar MS, Shah NM, Yusop SM (2021) Formulation and cost-effectiveness of fluid gels as an age-appropriate dosage form for older adults with dysphagia. Dysphagia.
- Sangnim T, Sriamornsak P, Singh I, Huanbutta K (2021) Swallowing gel for patients with dysphagia: A novel application of chitosan. Gels 7(3): 108.
- Mushtaque M, Muhammad IN, Hassan SMF, Ali A, Masood R (2020) Development and pharmaceutical evaluation of oral fast dissolving thin film of escitalopram: A patient friendly dosage form. Pak J Pharm Sci 33(1): 183-189.
- Moslemi M (2021) Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr Polym 254: 117324.
- Li DQ, Li J, Dong HL, Li X, Zhang JQ, et al. (2021) Pectin in biomedical and drug delivery applications: A review. Int J Biol Macromol 185: 49-65.
- Murata Y, Maida C, Kofuji K (2019) Drug release profiles and disintegration properties of pectin films. Materials 12(3): 355.
- Murata Y, Kokubo M, Miyashita M, Kawashima S (2003) Derivation of hydroxamic acid from pectin and its applications in colorimetric determination. Chem Pharm Bull 51: 897-898.
- Rupérez FJ, Fernández H, Barbas C (2002) LC determination of loratadine and related impurities. J Pharm Biomed Anal 29(1-2): 35-41.
© 2022 Yoshifumi Murata. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






