- Submissions

Full Text
Research & Development in Material Science
Recovery of Metallic Values in Smart Cards
Candeniz Uysal1, Selim Ertürk1, Fatma Arslan2* and Cüneyt Arslan1
1Department of Metallurgical and Materials Engineering, Istanbul Technical University, Istanbul, Turkey
2Department of Mineral Processing Engineering, Istanbul Technical University, Istanbul, Turkey
*Corresponding author: Fatma Arslan, Department of Mineral Processing Engineering, Istanbul Technical University, Istanbul, Turkey
Submission: October 29, 2021;Published: November 09, 2021

ISSN: 2576-8840 Volume 16 Issue 1
Abstract
Today, smart cards which include a small microchip are very widely used in our daily lives. The chief among these is credit cards. In addition, gift shopping card, phone card, sim card, hotel room card involves great proportions. They generally include many precious metals in their body, including noble metals, and semi-noble metals; predominantly gold, silver, copper, etc. The aim of this experimental work is to recover the metals from the smart cards by hydrometallurgical processes namely acidic leaching (HNO3 and Aqua Regia) followed by precipitation and cementation. Three different types of smart cards; credit cards supplied from different banks, telephone cards produced for Turk Telekom, and sim cards from three different mobile service providers, are used in the experimental work. As a result of this study, almost all copper, silver, and gold are recovered according to the experimental flowsheet proposed.
Introduction
Rapid global technological developments have led to the rising production of electronic
waste that presents both challenges and opportunities in its recycling. High metal values in
these types of wastes make them attractive for recovery. Treatment processes for metals
recovery from them are generally given in three groups as pyrometallurgy (roasting, smelting),
hydrometallurgy (leaching, separation), and biohydrometallurgy (leaching, adsorption).
Recycling of precious metals from WEEE (Waste Electrical & Electronic Equipment) typically
combined with mechanical pretreatment (manually dismantling, separation and shredding)
to segregate the valuable components followed by hydrometallurgical treatments through
leaching [1-4]. Aqua regia and mineral acids such as hydrochloric acid (HCl), nitric acid
(HNO3), and sulfuric acid (H2SO4) are used for the dissolution of PMs (Precious Metals)
from WEEE components [2]. Acidic leachates produced are further treated for the recovery
of PMs using conventional separation techniques, solvent extraction, membrane filtration,
cementation, ion exchange, adsorption, and so forth [3,5].
Smart cards are plastic cards which include a small microchip. This microchip has a
memory between 1 and 256 kilobytes of ROM and an operating system that has been written
on. The reason why these cards are named as smart cards is that they may contain and
process various kinds of data [6]. A smart card, in accordance with the programming of the
microprocessor’s, can be used as bank card, credit card, driver’s license, library membership
card, code card for electronic shopping, football club membership cards, electronic wallet,
etc., at the same time. Today, smart cards are very widely used in our daily lives. The chief
among these is credit cards. In addition, gift shopping card, phone card, sim card, hotel room
card involves great proportions. These microprocessors generally include many precious
metals in their body, including noble metals, and semi-noble metals; predominantly gold,
silver, copper, etc.
Metal ratio of a microprocessor has a big effect on its area of usage [7]. While the size of a
smart card (86x54x0.8mm) is regulated by the ISO-7810 standard, the smart card’s physical
characteristics, chip’s location on the credit card, the card material, magnetic stripe, signature region, the hologram on the card, picture, relief, etc. are defined by
the ISO-7816 standard [8,9]. Smart cards are divided into three
groups depending on the memory; memory only, security logic
memory, and memory with its own processor. Additionally, they can
be investigated as two groups, according to their connection type;
contact and contactless.
Today, smart cards have a big area of usage. According to the
data of the Interbank Card Center (BKM), as of the end of June 2021,
79.8 million credit cards, 141.5 million debit cards and 50 million
prepaid cards are used in Turkey [10]. In addition, according to the
research of We Are Social, the number of mobile phone users in
Turkey has reached 59 million. Of these, 77 percent, or 45 million,
use smartphones. Thus, it can be said that the number of SIM cards
in use is around 59 million [11]. Also, the amount of phone cards,
meal cards, etc., such as Sodexho and Ticket is nearly the same as
others. As of December 2017, there were 20.5 billion credit, debit,
and prepaid cards in circulation worldwide including global generalpurpose
cards [12]. With consumers worried about touching
surfaces during the coronavirus pandemic, the use of mobile
payments and contactless credit or debit cards has significantly
increased in this term [13]. The aim of this experimental work is
to recover the metals from the smart cards by using the similar
processes which have used in the recycling of WEEE’s.
Material and Method
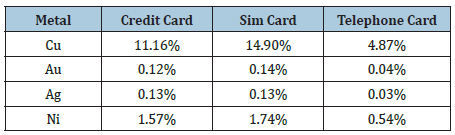
Three different types of smart cards; credit cards supplied from different banks, telephone cards produced for Turk Telekom, and sim cards from three different mobile service providers, are used in the experimental work. Every smart card is analyzed by wet and dry analysis methods to obtain their precious metal contents. Chemical analyses of the smart cards are shown in the Table 1.
Table 1: Chemical composition of smart cards.
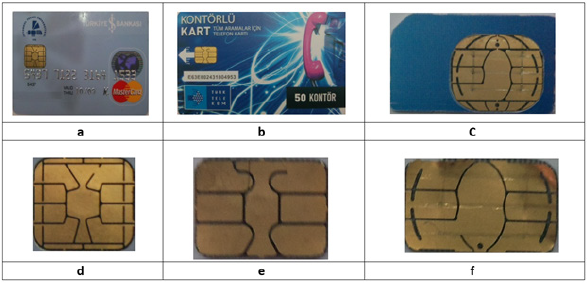
Chips are taken from the smart cards by a specially designed apparatus and weighted. After chips are dismantled from the smart cards, they were hold in hot water for 30 minutes to separate plastic part and chip. Weights of the raw smart card, taken part (chip and plastic body) and chip only are given in Table 2 and photographs of smart cards and chips are shown in Figure 1. Recycling of the plastic residue, from which the chip is removed is not a subject of this research.
Table 2: Weight of the various smart card parts.
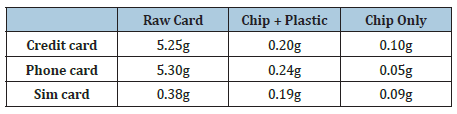
Figure 1 Photographs of the smart card and chips in different steps of the process (a) Credit card (b) Telephone card (c) Sim card (d) Credit card chip (e) Telephone card chip (f) Sim card chip.
Smart cards contain more than one metal (Au, Ag, Cu, Ni, and Zn) which exist all together. Their similar physical and chemical properties make it hard to separate them individually. At first step; silver, copper, and nickel are taken by HNO3 leaching. After that, copper and silver are separated by cementation. Silver is precipitated by copper powder from that solution. Afterwards copper is separated by zinc powder. Residue from HNO3 leaching is treated with aqua regia. Aqua regia is the only aqueous mixture which dissolves gold. Gold is precipitated from the acidic solution selectively. The most known chemicals for gold precipitation from acidic solutions are ferrous sulphate, sodium nitrite, sulphur dioxide, and sulphides [14]. Sodium metabisulphide is chosen as the precipitation agent. The practice of gold precipitation by sodium metabisulphide was developed in 1916 at the Nipissing mill to replace aluminum precipitation [15,16]. Flow sheet that has been applied on the experiments is given in Figure 2.
Figure 2 Flow sheet to recover metallic values in smart card chips.

Results
Nitric acid leaching and cementation experiments
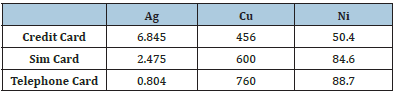
In the nitric acid dissolution experiments five different solutions (1-5%) are prepared and shredded chips are boiled for 30 minutes in that solutions separately. As a result, silver, copper, nickel, and zinc in the chip dissolve completely. After that, these metals are separated selectively by precipitation methods. Metallic ion concentrations after HNO3 leaching are given in Table 3.
Table 3: Metal concentrations after 5% HNO3 leaching (in ppm).
Aqua regia leaching and gold precipitation experiments
Aqua regia (a mixture of acids containing 3 units of HCl and 1 unit of HNO3) is a highly oxidizing acid in which gold dissolves easily. HNO3 leach residue is leached in 5% aqua regia solution and all of the metallic parts in chip dissolve after 15 minutes of reaction time. Results are given in Table 4.
Table 4: Metal concentrations after 5% aqua regia leaching (in ppm).

One of the most known chemicals for gold precipitation is the sodium metabisulphide. Precipitation reaction with sodium metabisulphide is given below:
2 AuCl3 + 3 Na2SO3 + 3 H2O → 2 Au + 6 HCl + 3 Na2SO4
1M sodium metabisulphide solution is added to the aqua regia solution after dissolution and stirred at 600-800rpm for 15 minutes. At the end It was found that gold is fully precipitated.
Conclusion
It can be seen from the experiments conducted in this work, silver and copper can be separated from nitric acid solutions by cementation successfully. Gold is precipitated by sodium metabisulphide from aqua regia solution with more than 90% for all the smart card types. Nickel in the solution can also be precipitated as nickel hydroxide. It can be concluded that, although a single smart card’s metallic value may seem to be very low, it could present enormous economic value when we look at the amount of its global production.
References
- Behnamfard A, Salarirad MM, Veglio F (2013) Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag 33(11): 2354-2363.
- Tuncuk A, Stazi V, Akcil A, Yazici EY, Deveci H (2012) Aqueous metal recovery techniques from e-scrap: hydrometallurgy in recycling. Miner Eng 25(1): 28-37.
- Hasegawa H, Barua S, Wakabayashi T, Mashio A, Maki T, et al. (2018) Selective recovery of gold, palladium, or platinum from acidic waste. Microchemical Journal 139: 174-180.
- Gouyon J, d'Orlyé F, Zimmerman J, Griveau S, Bedioui F, Varenne A (2020) Speciation and quantitation of precious metals in model acidic leach liquors, theoretical and practical aspects of recycling. Analytical and Bioanalytical Chemistry 412: 4595-4608.
- Umeda H, Sasaki A, Takahashi K, Haga K, Takasaki Y, et al. (2011) Recovery and concentration of precious metals from strong acidic wastewater. Mater Trans 52: 1462-1470.
- https://www.thalesgroup.com/en/markets/digital-identity-and-security/technology/smart-cards-basics
- (2007) Gedik Investment Securities Inc., Plastic Kart Smart Card Operating Systems Tic. Inc. (Plaskart) Company Report.
- https://www.iso.org/standard/70483.html
- https://www.iso.org/standard/14732.html
- https://www.diken.com.tr/turkiyede-kredi-karti-sayisi-798-milyona-yukseldi-kartla-odemeler-yuzde-42-artti/
- https://wearesocial.com/uk?s=how+many+cell+phones+are+in+use
- https://nilsonreport.com/upload/issues/1140_0321.pdf
- https://nrf.com/media-center/press-releases/coronavirus-leads-more-use-contactless-credit-cards-and-mobile-payments
- Demirkıran N, Ekmekyapar A, Künkül A, Baysar A (2007) A kinetic study of copper cementation with zinc in aqueous solutions. Int J Miner Process 82: 80-88.
- https://www.911metallurgist.com/blog/melting-gold-precipitate
- Demopoulos GP (2003) Nickel Hydroxide Precipitation from Aqueous Sulfate Media. JOM: The Journal of the Minerals, Metals & Materials Society 55(8): 42-46.
© 2021 Fatma Arslan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






