- Submissions

Full Text
Research & Development in Material Science
Effective Sonodegradation of Methylene Blue (MB) Organic Dye by Mil-88(Fe)/NaY/MnFe2O4 Nanocomposite Sonocatalyst in Aqueous Solution
Maryam Gholami1, Pourya Zarshenas2 and Meysam Sadeghi1*
1Department of Chemistry, Lorestan University, Khorramabad, Iran
2Faculty of Chemistry & Petroleum Sciences, Shahid Beheshti University (SBU), Iran
*Corresponding author: Meysam Sadeghi, Department of Chemistry, Lorestan University, Khorramabad, Iran
Submission: October 6, 2021;Published: October 20, 2021

ISSN: 2576-8840 Volume 16 Issue 1
Abstract
This research examined sonocatalytic degradation of Methylene Blue (MB) dye in the presence of MIL-88(Fe)/NaY/MnFe2O4 nanocomposite synthesized using the ultrasound assisted-hydrothermal route. Multiple identification techniques were utilized to investigate the MIL-88(Fe)/NaY/MnFe2O4 nanocomposite sonocatalyst involving FESEM, EDAX, FTIR, XRD and BET. The influences of various parameters like contact time, H2O2 concentration, initial MB concentration and sonocatalyst dosage were precisely studied. About 98.1% of MB dye degradation was achieved under the optimum parameter conditions i.e. at pH of 7, 25mg/L of initial MB concentration, H2O2 concentration of 4mM and 0.5g/L of MIL-88(Fe)/NaY/MnFe2O4 dosage within 60min. The enhancement of sonocatalytic activities can be related to the function of NaY zeolite as trap state for the electron. The scavenger tests outcomes demonstrated that the sono-generated hydroxyl radical (.OH) would play an important role in the MB degradation. Additionally, the MIL-88(Fe)/NaY/MnFe2O4 was quite stable since the efficiency of MB degradation gained in the four run was 93.7%.
Keywords: Sonocatalytic; Degradation; Methylene blue; MIL-88(Fe)/NaY/MnFe2O4; Nanocomposite
Graphical Abstract
Figure 1
Introduction
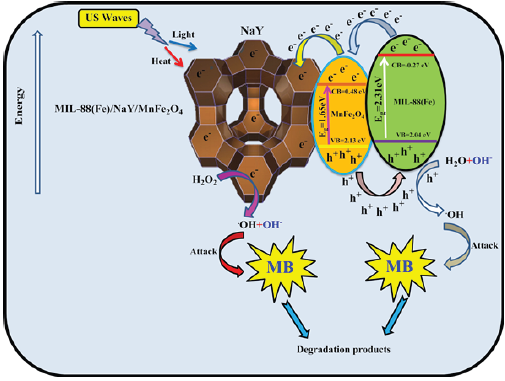
Today, organic dye sewages are a main source of water contamination [1,2]. Every year, the estimation demonstrates that up to 15% of dye sewages are evacuated into the surface and ground water supplies [3,4]. The exposure with these dye contaminants even at very low concentrations can be harmful to human and the environment [5]. For this reason, these toxic compounds must be removed from the environment. Recently, the various beneficial methodologies like adsorption, membrane filtration, electrochemical treatment, oxidation, ozonation, photocatalytic and sonocatalytic processes have been implemented for the removal of toxic organic dyes in the polluted aqueous solutions [6-11]. Among the aforementioned methods, the sonochemical process is considered as an effective route for the removal of these types of organic pollutants [12]. Plus, applying the ultrasound irradiation causes the production of the bubbles inside the reaction solution leading to the formation of high pressure and temperature approximately 1000atm and 5000K, respectively and subsequently the production of hot spots [13,14]. Eventually, the produced hot spots can in turn leading to the hydroxyl radical (.OH) and superoxide anion radical (.O2 -) originated from the breakdown of H2O and O2 molecules, respectively. Furthermore, it has been specified that the use of semiconductors catalysts along with the US/H2O2 system provides higher degradation performance in comparison to the H2O2 only and US only. Additionally, different types of catalysts, involving Cr-MIL-101@NiO/13X, InVO4/TiO2, NiGa2O4/CeO2, CoFe2O4/CdS, La/TiO2/Y have been used for the removal of organic dye pollutants via the sonodegradation processes [15-19]. Among these types of reported catalysts, Metal-Organic Frameworks (MOFs) are considered as new classes of porous compounds that have various applications due to their different properties such as catalysis, nanofluids, chemical sensing and so on [20-22]. On the other hand, the zeolites are introduced as a group of hydrated crystalline aluminosilicate involving alkali and alkaline earth metals [23]. These materials possesses unique properties such as high hydrothermal stability and biocompatibility, large pore volume and high surface area, which is suitable for its use as catalysis, adsorbents and so on [24-26]. Regarding the aforementioned explanations, it is explicitly known that combining the spinel MnFe2O4 nanoparticles with MIL-88(Fe) metal-organic framework in the NaY zeolite framework will produce a magnetically separable and superior nanocomposite catalyst that remarkably facilitate the hole and electron separation. Hence, in the presence work, the MIL- 88(Fe)/NaY/MnFe2O4, nanocomposite sonocatalyst was prepared using the ultrasound assisted-hydrothermal route. The as-prepared nanocomposite was fully characterized via the FESEM, EDAX, FTIR, XRD and BET analyses. Then, the above-mentioned nanocomposite catalyst was assessed for the effective sonodegradation of MB dye from aqueous solution in the presence of the ultrasound (US)/H2O2 system. Besides, the impacts of several parameters like contact time, H2O2 concentration, initial MB concentration and sonocatalyst dosage on the MB degradation were studied. Nevertheless, as far as we know, no study has been done on the sonodegradation reaction in the presence of the MIL-88(Fe)/NaY/MnFe2O4/US/H2O2 system in before.
Conclusion
To sum up, MIL-88(Fe)/NaY/MnFe2O4 was successfully synthesized by the ultrasound assisted-hydrothermal method. The results revealed that the sonocatalytic degradation of MB was obviously affected by contact time, H2O2 concentration, initial MB concentration and sonocatalyst dosage. The highest 60min at a pH of 7, 4mM of H2O2 concentration, 25mg/L of initial MB concentration and 0.5g/L of MIL-88(Fe)/NaY/MnFe2O4 dosage. The sonocatalytic process applying the MIL-88(Fe)/NaY/MnFe2O4, US (ultrasound) and H2O2 displayed great potential in treatment of MB. In addition, MIL-88(Fe)/NaY/MnFe2O4 could be applicable to the practical application since it showed high stability and recyclability. By this test, MIL-88(Fe)/NaY/MnFe2O4 nanocomposite illustrates a promising candidate for the sonocatalytic degradation in environmental remediation.
References
- Gusmão, Karla Aparecida Guimarães (2013) Adsorption studies of methylene blue and gentian violet on sugarcane bagasse modified with EDTA dianhydride (EDTAD) in aqueous solutions: kinetic and equilibrium aspects. Journal of Environmental Management 118: 135-143.
- Turki A (2015) Phenol photocatalytic degradation over anisotropic TiO2 nanomaterials: Kinetic study, adsorption isotherms and formal mechanisms. Applied Catalysis B: Environmental 163: 404-414.
- Harpreet SR (2005) Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Critical Reviews in Environmental Science and Technology 35(3): 219-238.
- Gupta VK (2009) Application of low-cost adsorbents for dye removal - A review. Journal of Environmental Management 90(8): 2313-2342.
- Sadettin S, Gönül D (2006) Bioaccumulation of reactive dyes by thermophilic cyanobacteria. Process Biochemistry 41(4): 836-841.
- Behnajady MA (2008) Ultrasonic degradation of Rhodamine B in aqueous solution: Influence of operational parameters. Journal of Hazardous Materials 152(1): 381-386.
- Zhang DQ, Zhang WL, Liang YN (2019) Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution-A review. Science of the Total Environment 694: 133606.
- Yalin Q (2014) RhB adsorption performance of magnetic adsorbent Fe 3 O 4/RGO composite and its regeneration through a fenton-like reaction. Nano-Micro Letters 6(2): 125-135.
- Sboui, Mouheb (2017) TiO2-PANI/Cork composite: A new floating photocatalyst for the treatment of organic pollutants under sunlight irradiation. Journal of Environmental Sciences 60: 3-13.
- Ayyappan CS, Bhalambaal VM, Sunil Kumar (2018) Effect of biochar on bio-electrochemical dye degradation and energy production. Bioresource Technology 251: 165-170.
- Hassan AM, El Jamal MM (2012) Kinetic study of the electrochemical oxidation of methylene blue with Pt electrode. Port Electrochimica Acta 30: 351-359.
- Ghodbane Houria, Oualid Hamdaoui (2009) Degradation of acid blue 25 in aqueous media using 1700kHz ultrasonic irradiation: ultrasound/Fe (II) and ultrasound/H2O2 Ultrasonics Sonochemistry 16(5): 593-598.
- Mason TJ, Phillip LJ (2002) Applied sonochemistry: the uses of power ultrasound in chemistry and processing. Wiley-Vch, Weinheim, Germany.
- Rajoriya S, Swapnil B, Virendra KS (2017) Degradation of reactive blue 13 using hydrodynamic cavitation: Effect of geometrical parameters and different oxidizing additives. Ultrasonics Sonochemistry 37: 192-202.
- Sadeghi Meysam (2019) Immobilization of Cr-MIL-101 over the NiO/13X zeolite nanocomposite towards ultrasound-assisted destruction of organic dyes in aqueous media. Journal of Water Process Engineering 32: 100946.
- YuLin M (2012) Sonodegradation and photodegradation of methyl orange by InVO4/TiO2 nanojunction composites under ultrasonic and visible light irradiation. Ultrasonics Sonochemistry 19(4): 883-889.
- Wang, Guowei (2020) Design and performance of a novel direct Z-scheme NiGa2O4/CeO2 nanocomposite with enhanced sonocatalytic activity. Science of the Total Environment 741: 140192.
- Atheel HA, Ahmad ZA, Norli I (2013) La loaded TiO2 encapsulated zeolite Y catalysts: investigating the characterization and decolorization process of amaranth dye. Journal of Engineering 2013.
- Farhadi Saeed, Firouzeh Siadatnasab (2016) CoFe2O4/CdS nanocomposite: Preparation, characterisation, and application in sonocatalytic degradation of organic dye pollutants. Chinese Journal of Catalysis 37(9): 1487-1495.
- Sadeghi M (2019) Immobilization of Cr-MIL-101 over the NiO/13X zeolite nanocomposite towards ultrasound-assisted destruction of organic dyes in aqueous media. Journal of Water Process Engineering 32: 100946.
- Díaz-Ramírez ML (2019) Partially fluorinated MIL-101 (Cr): from a miniscule structure modification to a huge chemical environment transformation inspected by 129 Xe NMR. Journal of Materials Chemistry A 7(25): 15101-15112.
- Sadeghi M, Saeed F, Abedin Z (2020) A novel CoFe2O4@Cr-MIL-101/Y zeolite ternary nanocomposite as a magnetically separable sonocatalyst for efficient sonodegradation of organic dye contaminants from water. RSC Advances 10(17): 10082-10096.
- Sadeghi M (2017) MnO2 NPs-AgX zeolite composite as adsorbent for removal of strontium-90 (90Sr) from water samples: Kinetics and thermodynamic reactions study. Materials Chemistry and Physics 197: 113-122.
- Dehghani, Modarres, Azadeh Tadjarodi, and Sanaz Chamani (2019) Synthesis and characterization of magnetic zeolite y-palladium-nickel ferrite by ultrasonic irradiation and investigating its catalytic activity in suzuki-miyaura cross-coupling reactions. ACS Omega 4(6): 10640-10648.
- Sadeghi, Meysam (2019) Synthesis of novel MnCo2O4/NaY zeolite nanocomposite adsorbent and its performance for Sr2+ ions removal from drinking water. Journal of Inclusion Phenomena and Macrocyclic Chemistry 93(3): 215-227.
- Hamed R, Seyed NA, and Sayed RH (2017) NaY zeolite as a platform for preparation of Ag nanoparticles arrays in order to construction of H2O2 sensor. Sensors and Actuators B: Chemical 248: 571-579.
© 2021 Meysam Sadeghi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






