- Submissions

Full Text
Research & Development in Material Science
Tailored Synthesis of PS@Ag@TiO2 Core-shell Nanocomposite for Photocatalytic Dye Degradation
Shanti Surong, Anindita Chakraborty and Himadri Acharya*
Centre for Soft Matters, Department of Chemistry, Assam University, India
*Corresponding author: Himadri Acharya, Centre for Soft Matters, Department of Chemistry, Assam University, Silchar-788011, Assam, India
Submission: July 22, 2021;Published: August 03, 2021

ISSN: 2576-8840 Volume 15 Issue 4
Abstract
Here, we describe a simple chemical method to synthesize multi component PS@Ag@TiO2 nanocomposites. The three-step synthetic approach comprises of synthesis of polystyrene (PS) particles, deposition of silver (Ag) nanostructure and coating of titanium dioxide (TiO2) nanoshells. As synthesized materials is characterised by microscopic and spectroscopic techniques. The size of the PS particles is obtained as approx. 200nm in diameter. Ag nanoparticles deposited PS particles are indicated by the surface plasmon band resonance in UV-vis study. To study the potential application, the photocatalytic activity of the PS@Ag@TiO2 nanocomposites is investigated in Methylene Blue (MB) dye degradation. Nanocomposite provides high photocatalytic degradation with a maximum efficiency of over 90% only in 30min.
Keywords: Polystyrene; Core-shell; Nanocomposite; Photocatalyst; Dye-degradation
Introduction
Nanocrystuctural titanium oxide (TiO2) has drawn intensive interests owing to its improved physical and chemical properties and promising technological applications in photocatalysis, photovolatic, displays,sensors and biolabel applications [1-4]. Anatase TiO2 has been widely investigated because of its easy synthesis and notable role in photocatalysis and photoelectron transfer [5,6]. However, the technological uses of TiO2 nanostructures has been recognised as one of the crucial issues due to its wide band gap (3.2eV) that require ultraviolet (UV) irradiation for photocatalytic activation and limits its application under visible light [7,8]. Thus, researchers has attempeted to develop TiO2 nanoparticle photocatalyst with enhanced photocatalytic activity over the past several years. A number of attempts has been made to develop TiO2 photocatalyst with visible light activity [9-12]. Thus, the development of TiO2 photocatalyst with visible light activity has attracted much attention over the past several years. Considerable efforts has been made by doping, surface modification, defects and depostion of noble metals. It was found that engineered TiO2 has high quantum yield and suppress the fast recombination of photogenerated electron-hole pairs [13-15].
The combination of semiconductor nanostructures with suitable metals e.g., Ag/TiO2, Au/TiO2 or Pt/TiO2 hybrids have shown excellent synergized photophysical properties [16-19]. The noble metal component promotes the interfacial charge-transfer processes by trapping the photo-induced charge carriers and exhibits enhanced photocatalytic performance. However, multicomponent systems with well defined morphology and composition is a key for the tailored function of the nanocomposite materials [20]. A facile, cost effective fabrication of integrated multicimponent system along with controlled properties are use for practical applications.
The fabrication of polystyrene-inorganic nanoparticle (core-shell) nanocomposite systems has been enormously studied due to their great advantages in electronics, catalysis, photonics, drug delivery and sensors etc [21,22]. Polystyrene (PS) particles are widely used as a core substrate in nanocomposite preparation because of their favorable properties, facile surface functionalization and size tunability. Moreover, the PS particles can stabilise the nanostructures and prevent from agglomeration. Core-shell nanoparticle based on PS particles with noble metal nanoparticles has common been accepted as effective nanocomposite materials and could be synthesized by various synthetic strategy including chemical reduction, polyol process, electroless plating etc [23-25]. Similarly, TiO2 nanostructure coated PS particles were synthesized by sol-gel method and layer-by-layer assembly [26,27]. The sol-gel method of nanostructure coating is widely used because of its simple synthesis and high efficiency. However, there is no earlier report on the synthesis of Ag@TiO2 shell on PS particles.
In this study, for the first timer we have synthesized a multicomponent composite is fabricated by step-wise growth of Ag and TiO2 nanostructure on PS particles. PS particles with uniform size and smooth surfaces were first synthesized, and then Ag nanostructure was coated on the surface of the PS nanoparticles. Finally, TiO2 nano shell was grown by sol-gel reaction, namely PS@Ag@TiO2 core -shell nanocomposite. The morphology of the nanocomposite was evaluated and applied in photocatalytic dye degradation.
Material and Methods
Chemicals and reagents
Styrene [(C8H9) 99%], azodoisobutylnytrile (AIBN) and silver nitrate (AgNO3) were purchased from Sigma-aldrich Co. Titanium bis acetylacetonate di isopropoxide (TDAA) (75% solution in 2ml of propanol was purchased from Merck specialities Pvt. Ltd. Sodium borohydride (NaBH4), polyvinylpyrrolidone (PVP) and sodium hydroxide (NaOH) were purchased Fisher Scientific Chemical Co. All the chemicals and solvent were ACS reagent grade. All chemicals were used as received without further purification. Deionized water was used in all the experimental processes.
Instruments
The morphology of the synthesized core-shell materials was investigated using transmission electron microscope (TEM. JEOL JEM-2100) operated at 200kV. The sample was prepared by drop casting the from ethanolic dispersion on to the carbon coated copper grid (200 mesh size) and dried overnight in vacuum. ZEISS-SIGMA Scanning Electron Microscope (SEM) was used and operated at 2.5kV. Samples for SEM was prepared on silicon wafer. By drop casting method and oven-dried overnight. The optical properties of core-shell nanocomposite were carried out by UVvisible spectrophotometry. The absorption spectra were recorded using JASCO V-670 spectrophotometer and 1 nm data interval in the range of 250-800nm.
Synthesis of Polystyrene (PS) particle
The PS particle was synthesized by dispersion polymerization of Styrene with AIBN initiator. First of all, the polymerization inhibitor is removed from the styrene by adding 5ml of styrene and 5ml of 10% NaOH solution in a reagent bottle and shakes vigorously to mix the two layers. The lower aqueous layer was removed by using separated funnel. Repeat the same washing process and then dried the styrene by adding granular CaCl2. For the dispersion polymerization of styrene, 0.3g of PVP stabilizer was dissolved in 20ml 0f isopropanol in a round bottom flask and heated to 70 °C. A solution of AIBN (25mg) pre-dissolved in 3g of dried styrene was added to the reaction flask under vigorous mechanical stirring and proceed the reaction for 24 hours before cooling to the ambient temperature. The PS particles were transferred from isopropanol to deionize water by repeated centrifugation and redispersion process and keep it for further used.
Preparation of nitro functionalized PS particle
The nitro-functionalized PS particle was synthesized via nitration of as prepared PS particle (1g) with nitric acid and sulfuric acid mixture (1:2v/v mixture) under slow stirring at 60 °C for 1h. Then the mixture was cooled to ambient temperature and washed repeatedly with 1(M) NaOH, water and ethanol till free from acid and dried in vacuum at 75 °C for overnight. Finally, the nitro functionalized PS particle are redispersed in distilled water (10wt%).
Preparation of silver nano particle coated polystyrene
25ml distilled water containing 0.5ml (10wt.%) of the asprepared PS particles was stirred for 30 mins with a magnetic stirrer at room temperature. Then, 10ml of freshly prepared 0.5mmol of AgNO3 (30mg) was added to the above solution and stirred for 30 mins. Finally, 5ml of 3mmol of NaBH4 aqueous solution was added drop wise into the solution mixture under ice cold condition and continued stirring for 20 hours. The white solution turned to brownish colour. The precipitate was collected by centrifugation at 5000rpm for 25 mins and washed 3 times with deionized water. The product was dried in vacuum at 60 °C for 2 hours.
Preparation of PS@ TiO2
The mixture of 10ml of isopropanol and 1ml of the as-prepared PS particles (10wt. %) was stirred for 30 mins at room temperature. A few drops of ammonia were added to the mixture maintaining pH value between 9-10. Afterward, 0.2ml of TDAA (Titanium (IV) bis acetylacetonate diisopropoxide) predissolved in 3ml of isopropanol was added to the above mixture part by part for three time. Then the mixtures were continuously stirred for about 20 hours at room temperature. Finally, it was centrifuged at 5000rpm for 25mins, and then the precipitates were washed with deionized water for about three time and dried in vacuum at about 50 °C.
Preparation TiO2@Ag@PS
In a 25ml RB flask 20ml of isopropanol was taken and 0.03g of as prepared PS @Ag@TiO2 emulsion was added to it. Then few drops of NH3 was added to maintain the pH value at 9-10. Afterward, 0.2ml of TDAA (Titanium (IV) bis acetylacetonate diisopropoxide) predissolved in 3ml of isopropanol was added to the above mixture part by part for three time. Then the mixtures were continuously stirred for about 20 hours at room temperature. Finally, it was centrifuged at 5000rpm for 25 mins, then the precipitate was washed with deionised water for about three time and dried in vacuum at about 50 °C.
Photocatalytic degradation of methylene blue
The photocatalytic activity of the nanocomposites was evaluated by performing the degradation of methylene blue (MB) as a representative dye. In a typical method, 1ml of (10wt.%) PS@ TiO2 and PS@Ag@ TiO2 nanocomposites were taken in 50ml 5ppm MB solution aqueous solution and stirred at room temperature under dark condition for 30 min to reach adsorption-desorption equilibrium. Then, 0.30mL of H2O2 (30%) was added to it and a small aliquot was withdrawn from the mixture at each given time interval and quantitatively analyzed using UV-vis spectrometer. After the concentration of the solution was determined by the absorption peak of the particles, the analyzed aliquot was quickly poured back into the reactor. The photocatalytic degradation ratio is recognized as It/Io where Io is the absorbance at equilibrium concentration and It is the absorbance of solution at time t. A 300W tungsten bulb was used as light source for photocatalytic degradation. The system was cooled by a fan to maintain the room temperature.
Result and Discussion
The synthetic approach towards PS@Ag@TiO2 core-shell nanocomposite is schematically illustrated in Figure 1. The PS particles are synthesized, and surface modified by nitro functionalization for Ag nanoparticle coating. Then sol-gel nanocoating of TiO2 shell on PS@Ag obtained from TDAA precursor. The amorphous core-shell nanostructure is spin coated to obtain thin film for photocatalytic reaction.
Figure 1: Schematic representation of fabrication of multicomponent PS@Ag@TiO2 core shell nanocomposite.
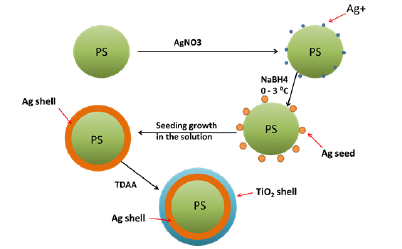
The structural morphology of PS, PS@Ag and PS@Ag@TiO2 is evaluated by TEM analysis. Figure 2a shows the TEM image of bare PS particle. It can be seen that the PS particles are uniform, with a smooth surface and a diameter of ca. 200nm. Figure 2b shows the TEM image of PS@Ag@TiO2 core-shell nanostructures. The surface of PS particle becomes rough and few Ag nanoparticles of below 4nm in size deposited on the PS particles. A thin layer of nanostructured Ag and overcoated TiO2 layers can be clearly observed at the edge of the particle in Figure 2c. The TiO2 shell deposited and completely covered the PS@Ag structure. A magnified image of an individual core-shell nanocomposite obtained by HRTEM is shown in Figure 2d reveals a typical core-shell structure with a lattice fringes of crystalline Ag nanostructure (is 0.23nm) corresponding to the fcc (111) facets. The TiO2 shell of approx. 10nm deposited on the Ag nanostructure corresponding to anatase TiO2. The SAED pattern in Figure 2e exhibits the typical diffraction rings indexed as (111), (200), (220), (311) and (222) planes.
Figure 2: TEM images of (a) PS particle (b,c) PS@Ag@TiO2 nanocomposite (b) HRTEM image showing the Ag and tiO2 nanostructure (e) SAED image.
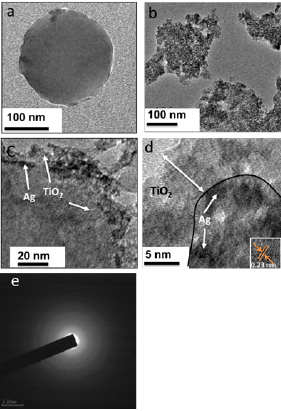
Figure 3a shows the optical micrograph of PS@Ag@TiO2 nanocomposites spin coated on the glass substrate. The particles are monodispersed and separated from each other. Figure 3b shows the SEM image of the core-shell particles. The coating of TiO2 can also be observed from the SEM image.
Figure 3: a. Optical micrograph of spin coated PS particles b. Sem image of PS@Ag@TiO2 core shell nanocomposite.
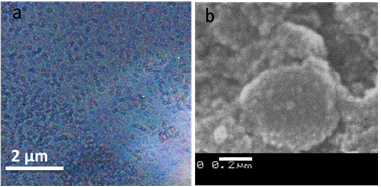
The characteristic absorption spectrum measured for bare polystyrene particle in Figure 4a is featureless. As a control experiment, TiO2 coated polystyrene particle in Figure 4b shows a weak UV-Vis band at around 270nm, which is characteristic band of TiO2 nanostructure. TiO2 particles coalesce into a network-like nanostructure on the surfaces of polystyrene particle and exhibit a weak band, instead of distinct peak. Figure 4c reveals a broad absorbance with an absorbance maximum at 403nm for Ag coated PS particle. This broad band is attributed to the localized surface plasmon resonance (SPR) of metallic Ag nanoparticles. The surface plasmon peak of Ag nanoparticles are red-shifted to 412nm when TiO2 deposited on the PS@Ag nanostructure surface. The increased interaction between Ag NPs with high k TiO2 is attributed to the red-shift of SPR of Ag NPs in the UV-vis spectra of PS@Ag@TiO2 core-shell structure. TiO2 with high band gap (~3.3eV) readily excite pairs of electrons and holes upon UV irradiation in the range of the SPR peak of Ag NPs and in turn can enhance the electric near field at the vicinity of the Ag NPs, leading to the red-shift of SPR peak [25].
Figure 4: UV-Vis spectra of a. Polystyrene particles b. PS@TiO2 particles c. nano structural Ag deposited PS particles d. PS@Ag@TiO2 core shell nanocomposite.
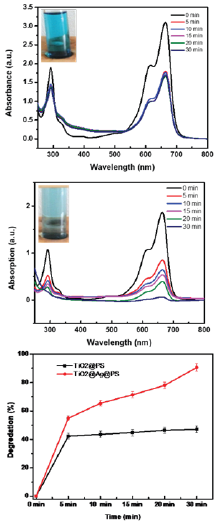
The photo-catalytic properties of PS@TiO2 and PS@Ag@TiO2 core shell nanocomposites respectively were evaluated by the degradation of methylene blue in hydrogen peroxide. Figure 4a & 4b shows the UV-vis absorption profile of MB dye upon photocatalytic reaction with PS@TiO2 and PS@Ag@TiO2 respectively in presence of H2O2. Figure 5a shows the photocatalytic degradation of MB dye in the presence of PS@TiO2. The absorption recorded after 5 min shows a significant decrease in the absorbance at λmax 665nm, which indicates the photocatalytic degradation. However, with further increase in time a mere decrease in dye concentration could be observed. Whereas, PS@Ag@TiO2 in Figure 5b exhibit a steady decrease in absorbance at λmax 665nm with time. However, there was no obvious degradation of R6G without catalyst or without H2O2 under the same conditions. Here, H2O2 acted as electron acceptors and could also decompose to produce •OH radicals to show a positive effect on the photocatalytic degradation reaction. Calculated amount of photocatalyst in methylene blue solution underwent photo-degradation under visible light irradiation due to photo-catalytic event of TiO2 nanoparticles by recombination of electron-hole pairs under irradiation. As a result, the characteristic absorption peaks of methylene blue drastically decrease in intensity with time.
Figure 5c shows the degradation percentage of MB dye with time, calculated from the relation (I0-I)/I0 (where, I0 is the MB absorbance maxima at t=0 and I is the absorbance maxima at a given reaction time, t). The plot shows 96% degradation for PS@Ag@TiO2 core-shell nanocomposite after 30min only compared to 48% for PS@TiO2. Such an increase in photo-catalytic activity for PS@Ag@ TiO2 core-shell nanocomposite may be explained by energy wasting step in the recombination of photo-generated electrons and holes. The electron-hole recombination of TiO2 nanostructure could be prevented by adding Ag nanostructure as electron acceptor to the system. The deposition of Ag nanoparticles to the PS particle and then by TiO2 network results the migration of the photo generated electrons from the surface of semiconducting TiO2 nanoparticle to Ag NPs of Ag/TiO2 hetero structure and thereby inhibit the recombination between electron-hole pairs.
Figure 5: a. Catalytic decomposition of methylene blue by PS@TiO2.
b. Catalytic decomposition of methylene blue by PS@ Ag@TiO2.
c. Degradation (%) plot of MB vs. Time.
Conclusion
PS@Ag@TiO2 photocatalyst was prepared by a three-step chemical approach and was characterized by microscopic and spectroscopic characterization. PS particles are of smooth surface with uniform size of ca. 200nm. Ag nanoparticles of <5nm in size deposited on the PS particles. The TiO2 nano shell of width approx. 20nm deposited on the PS@Ag nanostructure. The PS@Ag@TiO2 core-shell nanocomposite catalyst shows over 90% degradation only in 30 min of reaction time compared to 48% for PS @TiO2. The photocatalytic efficiency of PS@Ag@TiO2 nanocomposite catalyst enhances on Ag deposition is due to slow down in electron hole recombination process.
References
- Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107(7): 2891-2959.
- Reyes Coronado D, Rodriguez Gattorno G, Espinosa Pesqueira ME, Cab C, de Coss R, et al. (2008) Phase-pure TiO(2) nanoparticles: anatase, brookite and rutile. Nanotechnology 19(14): 145605.
- Bratovcic A (2020) A recent developments in photocatalytic water splitting by TiO2 Modified Photocatalysts. Res Dev Material Sci 14(1): RDMS.000827.
- Kondo K, Yoshikawa H, Awaga K, Murayama M, Mori T, et al. (2008) Preparation, photocatalytic activities, and dye-sensitized solar-cell performance of submicron-scale TiO2 hollow spheres. Langmuir 24(2): 547-550.
- Shchukin DG, Caruso RA (2004) Template synthesis and photocatalytic properties of porous metal oxide spheres formed by nanoparticle infiltration. Chem Mater 16(11): 2287-2292.
- Kim KT, Ali G, Chung KY, Yoon CS, Yashiro H, et al. (2014) Anatase titania nanorods as an intercalation anode material for rechargeable sodium batteries. Nano Lett 14(2): 416-422.
- Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: A historical overview and future prospects. Jpn J Appl Phys44(12):
- Im JS, Yun SM, Lee YS (2009) Investigation of multielemental catalysts based on decreasing the band gap of titania for enhanced visible light photocatalysis. J Coll Interf Sci 336(1): 183-188.
- Estruga M, Domingo C, Domenech X, Ayllon JA (2009) Low temperature N, Ndimethylformamide-assisted synthesis and characterization of anatase-rutile biphasic nanostructured titania. Nanotechnology 20(12): 125604-125611.
- Sayed M Saleh (2019) Metal oxide nanomaterials as photo-catalyst for dye degradation. Res Dev Material Sci 9(2): RDMS.000710.
- Joshi MM, Labhsetwar NK, Mangrulkar PA, Tijare SN, Kamble SP, et al. (2009) Visible light induced photoreduction of methyl orange by N-doped mesoporous titania. Appl Catal A Gen 357(1): 26-33.
- Li YZ, Zhang H, Hu XL, Zhao XJ, Han M (2008) Efficient visible-light-induced photocatalytic activity of a 3D-ordered titania hybrid photocatalyst with a core/shell structure of dye-containing polymer/titania. J Phys Chem C 112(38): 14973-14979.
- Chi B, Zhao L, Jin T (2007) One-Step template-free route for synthesis of mesoporous N-doped titania spheres. J Phys Chem C 111(17): 6189-6193.
- Nakamura R, Tanaka T, Nakato Y (2004) Mechanism for visible light responses in anodic photocurrents at N-doped TiO2 film electrodes. J Phys Chem B 108(30): 10617-10620.
- Savinkina E, Obolenskaya L, Kuzmicheva G (2015) Efficiency of sensitizing nano-titania with organic dyes and peroxo complexes. Applied Nanoscience 5: 125-133.
- Corno JA, Stout J, Yang R, Gole JL (2008) Diffusion-controlled self-assembly and dendrite formation in silver-seeded anatase titania nanospheres. J Phys Chem C 112(14): 5439-5446.
- Wang X, Yu JC, Ho C and Mak AC (2005) A robust three-dimensional mesoporous Ag/TiO2 nanohybrid film. Chem Commun (17): 2262-2264.
- Mikhail G (2018) Ceramic nanomaterials for high temperature applications. Res Dev Material Sci 3(3): RDMS.000563.
- Lee BY, Park SH, Lee SC, Kang M, Park CH, et al. (2003) Optical properties of Pt-TiO2 catalyst and photocatalytic activities for benzene decomposition. Korean J Chem Eng 20(5): 812-818.
- Manuel AP, Shankar K (2021) Hot electrons in TiO2-noble metal nano-heterojunctions: fundamental science and applications in photocatalysis. Nanomaterials 11(5): 1249.
- Chen LM, Chabu JM, Liu YN (2013) Bimetallic AgM (M=Pt, Pd, Au) nanostructures: synthesis and applications for surface-enhanced Raman scattering. RSC Adv 13(3): 4391-4399.
- Kityk IV, Ebothe J, Janczarek IF, Umar AA, Kobayashi K, et al. (2005) Nonlinear optical properties of Au nanoparticles on indium-tin oxide substrate. Nanotechnology 16(9): 1687.
- Li JM, Ma WF, Wei C, You LJ, Guo J, et al. (2011) Detecting trace melamine in solution by SERS using Ag nanoparticle coated poly(styrene-co-acrylic acid) nanospheres as novel active substrates. Langmuir 27(23): 14539-14544.
- Guo HX, Qin ZP, Wei J, Qin CX (2005) Synthesis of novel magnetic spheres by electroless nickel coating of polymer spheres. Surf Coat Technol 200: 2531.
- Kim DW, Lee JM, Lee JJ, Kang PY, Kim YC, et al. (2007) Formation and immobilization of silver nanoparticles onto chromia surface by novel preparation route involving polyol process. Surf Coat Technol 201(18): 7663-7667.
- Strohm H, Lobmann P (2005) Liquid-phase deposition of TiO2 on polystyrene latex particles functionalized by the adsorption of polyelectrolytes. Chem Mater 17(26): 6772-6780.
- Wang P, Chen D, Tang FQ (2006) Preparation of titania-coated polystyrene particles in mixed solvents by ammonia catalysis. Langmuir 22(10): 4832-4835.
© 2021 Himadri Acharya. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






