- Submissions

Full Text
Research & Development in Material Science
Disintegration Properties and Drug Release Profiles of Sodium Alginate Films Containing Rebamipide
Yoshifumi Murata*, Chieko Maida and Kyoko Kofuji
Faculty of Pharmaceutical Science, Hokuriku University, Japan
*Corresponding author: Yoshifumi Murata, Faculty of Pharmaceutical Science, Hokuriku University, Japan
Submission: March 15, 2021;Published: March 24, 2021

ISSN: 2576-8840 Volume 15 Issue 1
Abstract
Film Dosage Forms [FDs] prepared using water-soluble polymers are a strategy for drug delivery to local disease sites. In this study, we prepared FDs incorporating rebamipide as a model drug using various types of sodium alginates [Alg-Nas] as the film base via the casting method. Both the dissolution profile of each FD and the drug dissolution profile from the FD were investigated in a limited amount of physiological saline. A thin film incorporating rebamipide was obtained when either 2-4% low-molecular-weight Alg-Na or 1.5% high-molecular-weight Alg-Na containing chitin were used as the film base. When the FD was brought in contact with the test medium, the film matrix swelled and disintegrated. The disintegration profile of the FD differed according to the type of Alg-Na. However, the disintegration of the FD film matrix did not affect the drug dissolution rate. All materials used for the preparation of the FDs were selected according to their safety for oral administration; therefore, FDs may be an attractive therapeutic form for use in the oral cavity.
Keywords: Sodium alginate; Film dosage form; Rebamipide; Film disintegration profile; Drug release profile
Introduction
Sodium alginate [Alg-Na], an algal polysaccharide consisting of α-L-guluronate and β-D- mannuronate, is used as a food ingredient [1]. Some species of Alg-Na have been widely utilized as excipients in drug products and/or as impression materials in dentistry due to their polymeric properties and safe oral intake [2-4]. Alg-Na itself enacts a protective effect on the gastric mucosa; thus, it has been used as a medicine for the treatment of gastroesophageal reflux disease [5,6].
Rebamipide [RM] has been used as a gastroprotective drug against gastric ulcers [7,8]. RM is also used in patients with dry eye disease for its mucosal-protective effect, and its efficacy has been reviewed [9,10]. Recently, RM has been approved as a therapeutic agent for oral mucositis or ulcers by local application. For example, RM mouthwash has been used to treat oral mucositis induced by chemoradiotherapy and radiotherapy [11-13], and some attempts have been made to deliver RM to the local disease site [14,15]. A Film Dosage form [FD] is a thin film containing active compounds. FDs prepared using water-soluble polymers can quickly swell and disintegrate in a small amount of liquid, such as saliva. Therefore, water-soluble polysaccharides, such as Alg-Na, have been studied as base materials to prepare FDs, through which drugs can be efficiently delivered to local disease sites. We have reported Alg-Na as a useful polymer for preparing FDs, because thin films can be formed using this polysaccharide as a film base by simple methods that do not require dissolution in organic solvents [16].
In the present study, FDs containing RM were prepared via the casting method using some species of Alg-Nas as the film base. When FD is prepared with a water-soluble material such as Alg- Na, the disintegration profile of the film matrix is an important factor that characterizes the dosage form. Thus, the dissolution profiles of FDs were investigated using a colorimetric assay that measured the amount of Alg-Na in aqueous solutions [17]. The dissolution profiles of RM from the FDs were investigated in a limited amount of medium.
Experimental Materials
As the film base, we used two species of high-molecular-weight Alg-Na, Alg-A [300cps, Nacali Tesque Inc., Kyoto, Japan], Alg-B [500cps, Nacali Tesque Inc.] and two species of low- molecularweight Alg-Na, Alg-C [I-1G, Kimica Co., Tokyo, Japan], Alg-D [IL-1G, Kimica Co.]. RM was purchased from Tokyo Chemical Industry Co., Ltd. [Tokyo, Japan]. Chitin [Crab Shells, Nacali Tesque Inc.] and chitosan [fine powder, degree of deacetylation: 75-85%, Kimitsu Chemical Industries Co. Ltd., Tokyo, Japan] were used as additives to FD. Water-soluble carbodiimide, 1-Cyclohexyl-3-[2-morpholino ethyl] carbodiimide metho-p-toluenesulfonate [CMEC] were purchased from Aldrich Chemical Co. [Milwaukee, WI, USA]. All other chemicals were of reagent grade and were obtained from commercial sources.
FD preparation
FD was prepared as follows: 10ml of the base solution containing RM was dispersed in deionized water to prepare the film base solution. The mixture was thoroughly mixed by sonication and poured [3g each] into individual plastic Petri dishes [diameter, 54mm]. The dishes were kept at 40 °C for 24h, after which the circular films formed were transferred into a desiccator. The thickness was measured at 10 points on each film using a micrometer [CLM1-15QM; Mitutoyo, Kawasaki, Japan] with a set pressure of 0.5N. Measurements were taken using three films, and the mean thickness was calculated for each type.
Film disintegration test
A film was placed in a plastic dish, and 10ml of physiological saline preheated to 37 °C was added. The dish was then shaken [300rpm] in an incubator [SI-300; As One Co., Osaka, Japan] set at 37 °C. The medium [0.3ml] was periodically removed using a plastic syringe and filtered through a syringe-driven filter unit [pore size: 0.45μm]. An equal volume [0.3ml] of physiological saline at 37 °C was added to the dish in the incubator to maintain a constant volume. Aliquots [0.1ml] of the filtered solution were combined with 0.9ml of ion-exchanged water in the test tubes before thorough mixing with a vortex mixer. The amount of Alg-Na in each sample solution [ml] was measured using the method described below. Each test was performed in triplicate.
Alg-Na assay in a colorimeter
The reagent solutions used were 20mM HX in ion-exchanged water and 0.1M CMEC in 2% pyridine-HCl buffer [pH 5.0]. Aliquots [1ml] of HX and CMEC were added to 1ml of the sample solution, followed by vortexing. Each mixture was incubated at 40 °C for 20min, after which 20mM FeCl3 in 0.1M HCl [3ml] was added. The absorbance of the solution in a quartz cell [1cm light path] was measured at 480nm using a spectrophotometer [UV-1200; Shimadzu, Kyoto, Japan]. The absorbance was normalized to that of the blank reagent. For each test, a calibration curve was constructed using a fresh set of Alg-Na standards.
RM dissolution test
The sample solution was obtained using the method described in the film disintegration test section. Next, 80μL aliquots of the filtered sample solution were placed in micro test tubes [1.5ml], to which 720μL of methanol was added to precipitate the polysaccharide dissolved from the dosage form. Samples were mixed and centrifuged [7,700×g, 5min; H-1300; Kokusan Co., Saitama, Japan]. The supernatant was then injected into the HPLC column. Each test was performed in triplicate.
RM assay
The HPLC system [Hitachi Co., Tokyo, Japan] consisted of a pump [L-2130], UV-detector [L- 2400], autosampler [L-2200], and chromate-integrator [D-2500] connected to a packed column [150 mm×4.6mm, Cosmosil 5C18-MS-II, Nacalai Tesque Inc.]. To determine the RM concentration, the assay was performed at ambient temperature using a mobile phase consisting of 20mM potassium phosphate buffer [pH 6.8] and methanol [12:13] at a flow rate of 1.0mL/min [18]. The detector wavelength was set at 230nm.
Result and Discussion
As a base solution was poured onto a Petri dish to prepare FDs via the casting method, the viscosity of the solution was an important factor for casting [19]. Although both 1.5% high- molecular-weight Alg-Na and 2-4% low-molecular-weight Alg-Na could be cast, 2% high- molecular-weight Alg-Na solution could not be cast because of its high viscosity. The addition of RM to the base solution affected film formation by evaporating the solvent. As shown in Figure 1, FDs containing RM [0.75mg] were not obtained in all cases of 1.5% high-molecular-weight Alg-Na, such as Alg-A. Thin circular films containing RM formed when 2-4% low-molecular-weight Alg-Na was used as film base solutions. For example, an approximately 50μm-thick film was formed in the case of 2% Alg-C. FD was also obtained when 0.1-0.5% chitin or chitosan were added to 1.5% high- molecular-weight Alg-Na as an additive to the film base.
Figure 1: Images of film dosage forms prepared using the base solution containing RM.
[a] Alg-A, [b] 2% Alg-C, [c] 2% Alg-D, [d] 1.5% Alg-A containing 0.1% chitin, [e] 1.5% Alg-A containing 0.1%
chitosan.

When the FD was soaked in physiological saline at 37 °C, it swelled and then disintegrated, resulting in the release of Alg-Na into the medium. In the disintegration test, the amount of dissolve d Alg-Na was measured by a method that changed uronic acid within the polysaccharide to a hydroxamic acid derivative*. The disintegration profiles differed between the Alg-Na species used for the preparation of FD. The FDs prepared with low-molecularweight Alg-Na disintegrated quickly, as shown in Figure 2. For the FD prepared with 2% Alg-C, approximately 80% of the incorporated Alg-Na dissolved within 5min, and the total amount of the film base dissolved into the test medium within 10min. For the FD prepared with 2% Alg-D, 70% of the incorporated Alg-Na dissolved within 5min, and 88% of the film base dissolved into the test medium within 10min. In the case of FD prepared with 4% Alg-D, similar dissolution profiles were observed.
Figure 2:Dissolution profiles of Alg-Na from the
film dosage forms prepared with low-molecularweight
Alg-Na.
[Closed circle; 2% Alg-C, closed triangle; 2% Alg-D,
closed square; 4% Alg-D]
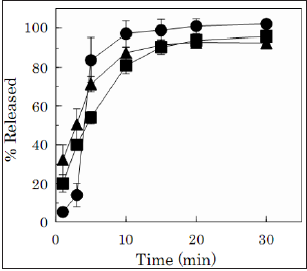
Figure 3: Dissolution profiles of Alg-Na from
the film dosage forms prepared with 1.5% highmolecular-
weight Alg-Na containing 0.1% additive.
[Open circle: Alg-A containing chitin, Open triangle:
Alg-B containing chitin, Open square: 4% Alg-A
containing chitosan]
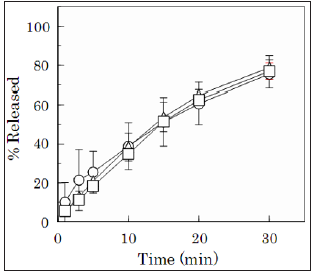
In contrast, the disintegration rate of FD prepared with highmolecular-
weight Alg-Na was slower than that of low-molecularweight
Alg-Na. Figure 3 shows the disintegration profiles of FDs
prepared with a high-molecular-weight Alg-Na-containing additive.
For example, for the FD prepared with 1.5% Alg-A containing 0.1%
chitin, 25% of the incorporated Alg-Na dissolved within 5min, and
75% of the film base was dissolved into the test medium within 30
min. Similar dissolution profiles were observed for both 1.5% Alg-B
containing 0.1% chitin and 1.5% Alg-A containing 0.1% chitosan.
As observed in the disintegration test, FDs prepared with Alg-
Na swelled, which led to disintegration; therefore, RM incorporated
in the FD was released from the preparation into the test solution.
Figure 4 shows the drug dissolution profiles of FDs prepared with
low-molecular-weight Alg-Nas. The amount dissolved into the
test solution at 5min was 0.48±0.05 mg of the drug incorporated
in the FD prepared with 2% AlgC. For the FD prepared with 2%
Alg-D, 0.73±0.01 mg of RM dissolved within 5min, and similar
RM dissolution profiles were obtained from the FDs prepared
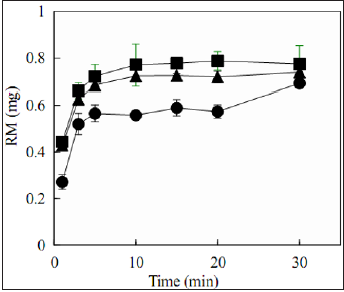
with 4% Alg-D. In the case of FDs prepared with high-molecularweight
Alg-Na- containing chitin, RM immediately dissolved
from the preparation. For example, RM (0.55±0.06 mg) dissolved
within 5min from the FD prepared with 1.5% Alg-A containing
0.1% chitin, as shown in Figure 5. The same drug dissolution
rates were also observed in the case of FD prepared with the base
solution containing 0.1% chitosan. These results show that the
disintegration of the FD film matrix did not affect the dissolution
rate of RM, and 0.5-0.7mg of RM dissolved in the medium within
10min in all cases. Similar drug release profiles were observed for
the FDs prepared with low-molecular-weight Alg-Na containing
0.1% chitin, as shown in Figure 6.
Figure 4: Release profiles of rebamipide from the
film dosage forms prepared with low-molecularweight
Alg-Na.
[Closed circle; 2% Alg-C, closed triangle; 2% Alg-D,
closed square; 4% Alg-D]

Figure 5: Release profiles of rebamipide from
the film dosage forms prepared with 1.5% highmolecular-
weight Alg-Na containing 0.1% additive.
[Open circle: Alg-A containing chitin, Open triangle:
Alg-B containing chitin, Open square: 4% Alg- A
containing chitosan]
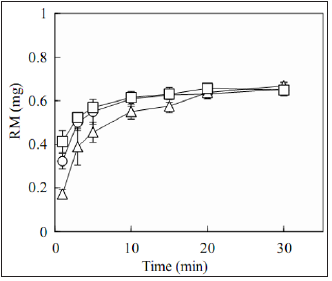
Figure 6: Release profiles of rebamipide from the
film dosage forms prepared with low-molecularweight
Alg-Na containing 0.1% chitin.
[Closed circle; 2% Alg-C, closed triangle; 2% Alg-D,
closed square; 4% Alg-D]
Conclusion
In this study, both the disintegration of FD prepared with Alg-Na and the drug release profile from the dosage form were investigated. The film matrix immediately swelled, even in a restricted amount of media, and disintegrated during the release of RM. All materials used for the preparation of FD were selected according to their safety for oral administration; therefore, FDs may be an attractive dosage form for use in the oral cavity.
References
- Ścieszka S, Klewicka E (2019) Algae in food: a general Crit Rev Food Sci Nutr 59(21): 3538-3547.
- Ye J, Ma D, Qin W, Liu Y (2018) Physical and antibacterial properties of sodium alginate-sodium carboxymethylcellulose films containing Lactococcus lactis. Molecules 23(10):
- Jin L, Qi H, Gu X, Zhang X, Zhang Y, et (2020) Effect of sodium alginate type on drug release from chitosan-sodium alginate-based in situ film-forming tablets. AAPS Pharm Sci Tech 21(2): 55.
- Cervino G, Fiorillo L, Herford AS, Laino L, Troiano G, et (2018) Alginate materials and dental impression technique: a current state of the art and application to dental practice. Mar Drugs 17[1]: 18.
- Leiman DA, Riff BP, Morgan S, Metz DC, Falk GW, et al. (2017) Alginate therapy is effective treatment for GERD symptoms: a systematic review and meta-analysis. Dis Esophagus 30(5): 1-
- Wilkinson J, Abd-Elaziz K, Den Daas I, Wemer J, Van Haastert M, et al. (2019) Two placebo- controlled crossover studies in healthy subjects to evaluate gastric acid neutralization by an alginate-antacid formulation (Gaviscon Double Action). Drug Dev Ind Pharm 45(3): 430-438.
- Jaafar MH, Safi SZ, Tan MP, Rampal S, Mahadeva S (2018) Efficacy of rebamipide in organic and functional dyspepsia: A systematic review and meta-analysis. Dig Dis Sci 63(5): 1250-1260.
- Han X, Jiang K, Wang B, Zhou L, Chen X, et (2015) Effect of rebamipide on the premalignant progression of chronic gastritis: A randomized controlled study. Clin Drug Investig 35(10): 665-673.
- Holland EJ, Darvish M, Nichols KK, Jones L, Karpecki PM (2019) Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul Surf 17(3): 412-423.
- Simsek C, Dogru M, Shinzawa M, Den S, Kojima T, et (2019) The efficacy of 2% topical rebamipide on conjunctival squamous metaplasia and goblet cell density in dry eye disease. J Ocul Pharmacol Ther 35(6): 350-358.
- Yokota T, Ogawa T, Takahashi S, Okami K, Fujii T, et al. (2017) Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II BMC Cancer 17(1): 314.
- Chaitanya B, Pai KM, Yathiraj PH, Fernandes D, Chhaparwal Y (2017) Rebamipide gargle in preventive management of chemo-radiotherapy induced oral Oral Oncol 72: 179-182.
- Akagi S, Fujiwara T, Nishida M, Okuda A, Nagao Y, et al. (2019) The effectiveness of rebamipide mouthwash therapy for radiotherapy and chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: A systematic review and meta-analysis. J Pharm Health Care Sci 5: 16.
- Takeuchi I, Kamiki Y, Makino K (2018) Therapeutic efficacy of rebamipide-loaded PLGA nanoparticles coated with chitosan in a mouse model for oral mucositis induced by cancer Colloids Surf B Biointerfaces 167: 468-473.
- Takeuchi I, Togo C, Makino K (2019) Rebamipide-containing film using chitosan and HPMC for oral mucositis induced by cancer chemotherapy, Anticancer Res 39(12): 6531-6536.
- Murata Y, Kanemaru H, Tsushima M, Maida C, Kofuji K (2018) Film dosage forms prepared with alginate for oral candidiasis Res Dev Material Sci 4(3): 591.
- Murata Y, Maida C, Kofuji K (2019) Drug release profiles and disintegration properties of pectin Materials 12(3): 355.
- Sonawane S, Gide P (2011) Optimization of forced degradation using experimental design and development of a stability-indicating liquid chromatographic assay method for rebamipide in bulk and tablet dosage Sci Pharm 79(1): 85-96.
- Murata Y, Isobe T, Kofuji K, Nishida N, Kamaguchi R (2010) Preparation of fast dissolving films for oral dosage from natural Materials (Basel) 3(8): 4291-4299.
© 2021 Yoshifumi Murata. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






