- Submissions

Full Text
Research & Development in Material Science
A Recent Developments in Photocatalytic Water Splitting by TiO2 Modified Photocatalysts
Amra Bratovcic*
Department of Physical Chemistry and Electrochemistry, Faculty of Technology, University of Tuzla, Bosnia and Herzegovina
*Corresponding author: Amra Bratovcic,Department of Physical Chemistry and Electrochemistry, Faculty of Technology, University of Tuzla, Bosnia and Herzegovina
Submission: August 10, 2019;Published: August 25, 2020

ISSN: 2576-8840 Volume 14 Issue 1
Abstract
As populations grow, global energy consumption in the next 30 years is predicted to rise by nearly 50%. Nowadays and many years before, the most energy worldwide is provided by fossil fuel which leads to severe pollution and contributes to the greenhouse effect. Hydrogen is the most ideal alternative clean energy, but currently, there is no significant hydrogen production from renewable sources. Hence, there is an urgent need for the development of new photocatalysts which will allow a water splitting for hydrogen production. The photocatalytic water splitting using TiO2 offers a promising approach for clean, low-cost, and environmentally friendly production of hydrogen as a sustainable fuel. This paper reviews some recently used methods of synthesis such as hydrothermal, rapid breakdown anodization method, impregnation method, and sol-gel synthesis for the preparation of modified TiO2 materials. These methods of synthesis provide the production of ultra-thin mesoporous TiO2 nanosheets, nanorods, and nanotubes as well as heterojunction structures. Some investigations show that introduction of Ti3+ atomic defects is beneficial for the photocatalytic water splitting for hydrogen generation. Some progress has been achieved by hetero coupling the two or more semiconductors. There is experimental evidence that in the presence of alcohol as a sacrificial agent, H2 production rates decreased from a higher number of hydroxyl groups i.e. in order 3>2>1. The H2 generation is also larger when TiO2 is modified with the addition of small quantity of metal nanoparticles such as Pt, Pd, and Ni. One study has shown that the samples sensitised with Pt nanoparticles were superior to Pd and Ni modified TiO2, the other has shown that the co-catalyst activity followed the order Pd>Pt≈Au.
Keywords: Photocatalysis; Water splitting; Hydrogen production; Modified TiO2; Nanoparticles
Abbreviations: °C: Degrees Celsius; NPs : Nanoparticles; NTs: Nanotubes; EC: European Commission; IRENA: International Renewable Energy Agency; IPCC: Intergovernmental Panel on Climate Change; EIA: Energy Information Administration; CB: Conduction Band; VB: Valance Band; CBE: Conduction Band Energy; Eg: Band Gap Energy; ΔG: Gibbs Free Energy
Introduction
As populations grow, global energy demands together with their potential environmental impact are expected to increase even more in the coming years. According to the U.S. Energy Information Administration (EIA), between 2018 and 2050, the world energy consumption is predicted to rise by nearly 50% [1]. In 2010, fossil fuels provided about 80% of all primary energy worldwide [2]. The combustion of fossil fuels lead to severe pollution and contributes to the greenhouse effect. Climate is a main driver for hydrogen in the energy transition. Limiting global warming to below 2 degrees Celsius (°C) requires that CO2 emissions decline by around 25% by 2030, from 2010 levels, and reach net zero by around 2070 (IPCC, 2018) [3]. For a reasonable likelihood to stay below 1.5 °C of warming, global net anthropogenic CO2 emissions should decline by around 45% by 2030, from 2010 levels, reaching net zero by around 2050 [3].
The world must therefore balance the role of energy in social and economic development with the need to decarbonise, reduce our reliance on fossil fuels, and transition towards lower-carbon energy sources. Hence, there is an urgent need for the development of clean and sustainable sources of energy. Hydrogen is considered to be the most ideal alternative clean energy because of its high calorific value, zero pollution, and storability, and it is widely considered to be the future clean energy carrier in many applications, such as environmentally friendly vehicles, domestic heating, and stationary power generation. Today, around 95% of all hydrogen is generated from natural gas and coal resulting in the release of 70 to 100 million tonnes CO2 annually in the EU (EC, 2020) [4].
For hydrogen to contribute to climate neutrality, it needs to achieve a far larger scale and its production must become fully decarbonised. Around 5% is generated as a by-product from chlorine production through electrolysis. In the iron and steel industry, coke oven gas also contains a high hydrogen share, some of which is recovered. Currently there is no significant hydrogen production from renewable sources. However, this may change soon. Currently, hydrogen is used mostly in oil refining and to produce ammonia [5].
Water splitting and alcohol photo-reforming using semiconductor photocatalysts and sunlight are promising future technologies for H2 production. Water splitting plays an important role in producing of green and clean energy in the form of H2 for the next generation. The generation of chemical fuels from water and sunlight is one of the key scientific challenges for the 21st century. Nowadays, hydrogen is being produced in large quantities for industrial and commercial purposes. Yet only 5% of commercial H2 is produced through electrolysis of water and the rest entirely depends on the fossil fuel sources [6,7].
Since the first discovery of photocatalytic water splitting on a TiO2 electrodes observed by Fujishima [8] researchers have focused on it using a variety of semiconductors such as TiO2 [9], graphitic-carbon nitride [10], and CdS [11]. Among them, TiO2 attracted much attention of many research groups and quickly became the most studied and used semiconductor for photocatalysis [12-15]. TiO2 is considered as a promising semiconductor because of the advantages such as low cost, harmlessness, resistance to photo-induced corrosion and ease of handling, but its performance is still limited due to its large bandgap, approximately 3.2eV for the anatase phase in powders [16-19].
Thus, to address these difficulties, it is extremely desirable to search for new materials that could split water by absorbing visible light. In this regard, modifications in titania for accelerating photocatalytic hydrogen generation from water is a favorable approach. Various attempts have been carried out to modify TiO2 in order to present a response with visible light [20]; some techniques include the doped with metals [21] and non-metals [22], as well as several sensitization methods with dyes [23], quantum dots [24], and coupling of other semiconductors (as a heterojunction composite) [25,26], among others.
TiO2 has a wide range of applications and it was extensively discussed in previously published papers for wastewater treatment such as photocatalytic degradation of organic compounds, as well as micro- and nano-plastics, etc. [27-31]. During photocatalytic water splitting the hydrogen is produced. Many research studies have shown that higher amount of hydrogen is produced in the presence of an electron donor such as alcohol (methanol, other primary alcohols, or polyols such as ethylene glycol) i.e. in the water-alcohol solution and differently dopped TiO2 photocatalysts [32].
Alcohol as a sacrificial agent has lower splitting energy than water. Many published papers mention that “water splitting” are actually related to redox of the additives or probably corrosion and therefore do not deal with water splitting [33,34]. In a last decade, different type of nanomaterials and its composites have been developed and used for a wide range of applications which among others include photocatalytic reactions and hydrogen production and fuel cells [35-39].
Figure 1 shows a plot of number of publications about “photocatalytic water splitting” versus publication year [ScienceDirect on 23.07.2020]. The figure shows increasing numbers of publications in last ten years which indicate very high interest in photocatalytic water splitting from year to year. Nevertheless, hydrogen production systems based on heterogeneous photocatalysis are in constant development and change, as researchers look for more environmentally friendly photocatalytic processes.
Figure 1: Number of publications about “photocatalytic water splitting” versus publication year

Methods of synthesis
In this section an overview of different methods of synthesis such as hydrothermal approach, sol-gel method, rapid breakdown anodization will show different shapes of TiO2 and its photocatalytic properties.
Hydrotermal synthesis of ultra-thin mesoporous TiO2 nanosheets: The study carried out by Li et al. [40] offers inspiration for designing efficient photocatalysts and provides valuable insights towards defect engineering in photocatalysts. They were successfully synthesized ultra-thin mesoporous anatase TiO2 nanosheets for hydrogen evolution from water splitting. The synthetized mesoporous TiO2 has optimum aperture almost keeps around 2nm and the surface area is around 152.938m2/g and pore volume 0.162cm3/g.
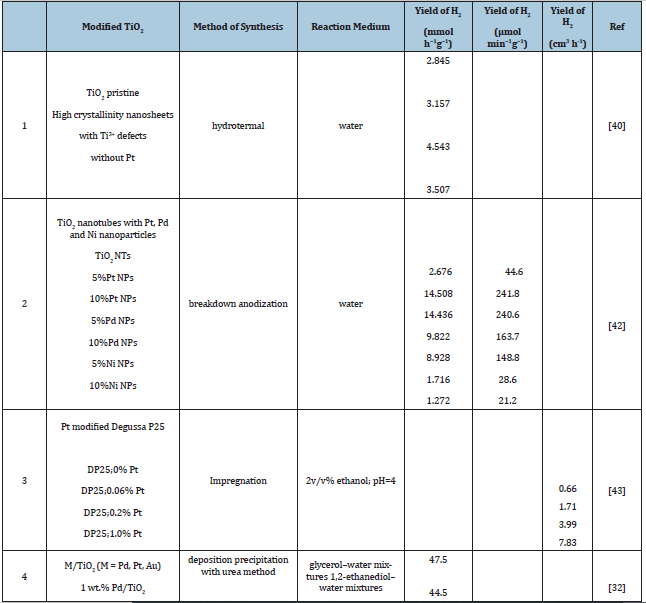
Ultra-thin mesoporous TiO2 nanosheets with high crystallinity have been obtained through a facile hydrothermal approach with the assistance of chlorine ions, which is not only beneficial for obtaining stable mesoporous TiO2 with high crystallinity, enlarging surface area to 178.496m2/g and the pore volume to 0.201cm3/g. Although the surface area and pore size have less decrease after ethylene glycol treatment, the pore volume enhances. It thus demonstrated that this facile Cl ions assisted method could lead to obtaining stable anatase TiO2 nanoparticles with high crystallinity while retaining or even enlarging surface area. Ti3+ atomic defects in the ultra-thin mesoporous TiO2 play a crucial role in suppressing the recombination of electron-hole pairs and enhancing the photocatalytic H2 production rate. They explored the photocatalytic activity of the mesoporous TiO2 towards photocatalytic water splitting under simulated solar light irradiation without filter. The experimental results has shown that H2 generation yield of pristine mesoporous TiO2 is only 2.845mmol h−1g−1, while it reaches to 3.157mmol h−1g−1 in high crystallinity TiO2 and 4.543mmol h−1g−1 after further introducing Ti3+ defects. As expected, the increase rate of TiO2 with Ti3+ defects reach 59.66%, which far exceeds that of others. It further confirms that the introduction of Ti3+ atomic defects is beneficial for the photocatalytic water splitting for hydrogen generation. They found a H2 yield of 3.507mmol h−1g−1 without the Pt particle deposition indicating that Ti3+ point defects are beneficial for photocatalysis performance. They conclude that removing excess unfavourable defects and reintroducing advantageous ones is a promising way to improve photocatalysis performance.
Dosado et al. [41] were prepared a series of titania nanorods with different phase compositions and surface areas by calcination of hydrogen titanate (H2Ti3O7) nanotubes at temperatures (150-1000 °C). Sodium titanate (Na2Ti3O7) nanotubes were synthesised by alkaline hydrothermal treatment of the anatase TiO2 powder. Hydrogen titanate (H2Ti3O7) nanotubes were synthesised from the Na2Ti3O7 nanotubes by ion exchange. Briefly, the sodium titanate nanotubes were dispersed in 1M HCl (500mL) for 2h. Gold nanoparticles were deposited on the H2Ti3O7 nanotubes, TiNTx (x=calcination temperature=350-1000) and Degussa P25 supports at nominal Au loading of 0.5, 1.0, 1.5 or 2.0 wt.% using the deposition-precipitation with urea method. The results of their research showed that 0.5 wt.% Au/TiNT600 photocatalyst has excellent H2 production activity in all the alcohol-water systems, performing similarly to a 1.5 wt.% Au/P25 reference photocatalyst. For both the 0.5 wt.% Au/TiNT600 and 1.5 wt.% Au/P25 photocatalysts, H2 production rates decreased in the order triol (glycerol) > diol (1,2-ethanediol ≈ 1,2-propanediol) > ethanol > 1-propanol. Good correlations were found between the H2 production rates and alcohol properties such as the number of hydroxyl groups, polarity, or standard oxidation potential.
Rapid breakdown anodization method: Manovah et al. [42] have prepared TiO2 nanotubes powders via rapid breakdown anodization sensitized with Pt, Pd and Ni nanoparticles (NPs) for photocatalytic water splitting i.e. for photogeneration of H2. The samples sensitized with Pt NPs were superior to Pd and Ni sensitized TiO2 NTs powders. Noticeably, the smaller the quantity of the NPs loaded over the TiO2 NTs better the photogeneration of the H2 They have found that the H2 generation is larger with the addition of metal NPs and moreover lower quantity deposits produced better results. Pt NPs addition was better among the group in terms of H2 generation over the other metals of the same group. The Pt/TiO2 NTs with 5% metal sensitization generated H2 with a specific release rate of 241.8μmol g−1 min−1 [42].
Impregnation method: Salas [43] in his PhD dissertation demonstrates that hydrogen can be produced photocatalytically using a modified Degussa P25 (TiO2)-Pt photocatalyst in a slurry medium under near-UV irradiation and having ethanol as a sacrificial reagent (hole scavenger). The system employed for the photocatalytic hydrogen generation was the Photo-CREC Water II Reactor. This unit was used with a specially designed H2 collector tank and a BLB Lamp that permits the entire use of the near-UV irradiation spectrum.
The modified DP25-Pt photocatalyst was prepared using the incipient wetness impregnation technique. The Pt modified photocatalyst exhibited a 2.73eV reduced band gap. The use of 2v/v% ethanol as a sacrificial reagent enabled producing significant hydrogen amounts with the simultaneous formation of CH4 and C2H6 by products. It is proven that hydrogen formation in the presence of ethanol is a function of water solution pH and Pt loading on the TiO2 photocatalyst. Concerning energy efficiencies, it was observed that the best results obtained are at pH=4 with 7.9% quantum yield for hydrogen production.
In Table 1 is summarized the influence of method of synthesis and modification of TiO2, reaction medium on H2 yield.
Sol-gel synthesis of TiO2 thin films: Thin titanium dioxide (TiO2) films show good electrochemical and photocatalytic properties for photocatalysis applications. The textural properties such as thickness, surface roughness, grain or particle size, pore size distribution and porosity of the TiO2 films influence on photocatalytic activity [44].
Table 1: Influence of method of synthesis, modification of TiO2 and reaction medium on H2 yield.
These textural properties depend on the sol properties (sol reactivity, viscosity, water-to-alkoxide ratio, precursor concentration, complexing agent, surfactant) [45,46]. The sol properties can be controlled by varying its composition. Spin-coating of TiO2 sol-gel is the common deposition method used to coat TiO2 thin films on the glass substrates due to its low cost, experimental simplicity, easy scale-up ability [47]. In the sol-gel method, hydrolysis of metal alkoxides occurs first followed by the polycondensation of hydroxyl and/or alkoxy groups thus forming the oxide polymer network of TiO2 via Ti-O-Ti route [48]. Two main reactions in the sol-gel process are hydrolysis of titanium tetraisopropoxide (TTIP) with water and condensation of hydrolyzed TTIP.
The faster hydrolysis of the TTIP with water results in the rapid precipitation of hydrolyzed TTIP due to high reactivity, which in turn leads to non-homogeneity in the films. Therefore, the hydrolysis rate needs to be controlled in order to avoid precipitation and to achieve sol-gels with desired properties to obtain highly homogeneous TiO2 thin films [49,50].
Effect of sacrificial agent on water splitting
Many research studies carried out in the last thirty years have shown that direct photocatalytic water splitting to H2 and O2 using a wide range of semiconductor photocatalysts is too low to justify industry uptake. H2 production rates can be increased by several orders of magnitude using renewable sacrificial agents such as ethanol or glycerol, though further work is necessary to realise target H2 production rates of 2-3mmol gcat -1 min-1 under direct sunlight that are needed to attract industry interest.
For example, in a Pt/TiO2-water-methanol system, methanol is oxidized by means of the photogenerated valence band (VB) holes on the metal-decorated photocatalyst thus permitting the reduction of water to hydrogen by photogenerated electrons in the conduction band (CB). Such sacrificial reagents react irreversibly with the photogenerated holes and enhance consequently the electron/hole separation. This concept could be used for the photocatalytic production of hydrogen with simultaneous degradation of pollutants or what is known as photo reforming [7].
In 2015 Al Azri et al. [32] found that M/TiO2 (M=Pd,Pt, Au) photocatalyst activity for H2 production depends on both the metal co-catalyst (M) and the reaction medium (different alcohol-water mixtures). They were prepared photocatalysts using Degussa P25 TiO2 at metal loadings of 0.5 and 1wt.% for Pd, 1wt.% for Pt and 1wt.% for Au. The activities of the M/TiO2 photocatalysts for H2 production were evaluated in a wide range of alcohol-water mixtures (alcohol concentration 10 vol. %) under UV (365nm, 5mWcm-2). H2 production rates in the alcohol-water mixtures were dependent on (i) the metal co-catalyst; (ii) the co-catalyst loading; and (iii) the alcohol type. Co-catalyst activity followed the order Pd>Pt≈Au. Metal co-catalyst particle size was not important for achieving high hydrogen production rates in the M/TiO2 systems, at least within the size range 1-6nm. The highest H2 production rates were achieved for the 1wt.% Pd/TiO2 photocatalyst in glycerol-water mixtures (47.5mmol g-1 h-1) and 1,2-ethanediol-water mixtures (44.5mmol g-1 h-1). H2 production rates decreased in the order glycerol > 1,2-ethanediol > 1,2-propanediol > methanol > ethanol > 2-propanol > tert-butanol ≫ water. For each M/TiO2 photocatalyst, correlations were established between the rate of H2 production and specific alcohol properties, especially alcohol polarity and the exponential of the alcohol oxidation potential.
Heterojunction structures
In order to prevent the rapid charge carrier recombination and to isolate the redox sites on the catalytic surface separately, it is essential to spatially separate the electrons and holes in different compartments which can be accomplished by heterocoupling the two or more semiconductors. Recently heterojunctions formed between two solid materials have attracted more attention, including semiconductor-semiconductor S-S, semiconductor-metal S-M, and semiconductor-carbon S-C (carbon nanotubes, graphene, etc.) heterojunctions [51].
Some progress has been achieved in the use of complex oxides (Rh-SrTiO3, [52] PbBi2Nb2O9, [53] Sr1-xNbO3 [54] and oxynitrides SrNbO2N, [55] LaTiO2N, [56] and TiON [57]) for overall water splitting under visible light but these materials are either difficult to synthesise in pure form, or the efficiencies reported are comparatively moderate. However one of the most promising, but lesser studied oxynitrides for overall water splitting under visible light is TaON (CBE at -0.3 V vs. NHE, pH 0), which has recently received much attention through its incorporation into a heterojunction, for example with CaFe2O4, [58] N-doped TiO2 [59] and Cu2O [60]. It is clear that transition metal oxynitrides have great potential in the future for utilization in water splitting systems, however more research into this emerging family of photocatalyst materials is required, particularly their incorporation into junction architectures.
During the composite formation, defect density at the interface minimizes and the new functionalities arising at the interfacial structure that is normally not attained in their respective single phases. The coupling of ZnO with TiO2 overlayer avoids the photo corrosion of ZnO and also improves the light absorption properties of ZnO-TiO2 as ZnO owns high absorption efficiency compared to pure TiO2 [61]. Electron transfer takes place from ZnO to TiO2 because electron derived from ZnO is more negative, while inter component hole transfer may not be significant as VB edge position of both ZnO and TiO2 are almost same. Along with the latter, absorption range of this composite is not altered as both are wide gap in nature. Therefore, ZnO-TiO2 composite may not be beneficial in all the cases. However, pronounced blue shift up to 3.8eV was noticed for this composite when fabricated through combined sol-gel technique and dip coating on the quartz substrate [62].
Mechanisms of semiconductor photocatalytic water-splitting
TiO2 is a semiconductor consists of valance band (VB) and conduction band (CB). Energy difference between these two levels is said to be the band gap, Eg. Without excitation, both the electrons and holes are in valence band. When semiconductors are excited by photons with energy equal to or higher than their band gap energy level, electrons receive energy from the photons and are thus promoted from VB to CB if the energy gain is higher than the band gap energy level.
The photo-generated electrons and holes can recombine in bulk or on surface of the semiconductor within a very short time, releasing energy in the form of heat or photons. Electrons and holes that migrate to the surface of the semiconductor without recombination can, respectively, reduce and oxidize the reactants adsorbed by the semiconductor. The reduction and oxidation reactions are the basic mechanisms of photocatalytic hydrogen production. Thermodynamically, the water splitting reaction is an uphill process, i.e. is an endothermic reaction requiring a minimum energy of 1.23eV because the Gibbs free energy change for the reaction is ΔG°=237.2kJ mol-1 or 2.46eV per molecule of H2O, and therefore requires high overpotentials [63]. Nature itself demonstrates an efficient strategy to utilise solar irradiation (near unity quantum yield) by spatially separating electrons and holes in wireless photosynthesis reactions. The process of water splitting can be envisaged as two half reactions: water oxidation, and secondly, proton reduction to hydrogen fuel.
For hydrogen production, the CB level should be more negative than hydrogen production level (EH2/H2O) while the VB should be more positive than water oxidation level (EO2/H2O) for efficient oxygen production from water by photocatalysis [7]. In the case of photocatalytic water splitting, a catalyst absorbs photon energy and consequently, electrons are transferred from its valence band to its conduction band. If its band gap is large enough, above that needed for water splitting (1.23eV), and its band edges meet the thermodynamic requirement for the charge transfer to occur, then in principle, excited electrons can reduce hydrogen ions and holes can oxidize oxygen anions [64].
The photocatalytic hydrogen production from water splitting by TiO2 is shown in Figure 2. Theoretically, all types of semiconductors that satisfy the above-mentioned requirements can be used as photocatalysts for hydrogen production. Having strong catalytic activity, high chemical stability and long lifetime of electron/hole pairs, TiO2 is the most widely used photocatalyst.
Figure 2: Mechanism of TiO2 photocatalytic water splitting for hydrogen production.

According to the proposed mechanism when metal nanoparticles (NPs) are used for sensitizing TiO2 upon exposure to UV light of 365nm, TiO2 NPs form electrons and holes [65]. The electrons first move to the CB of the TiO2 and from there it interacts with the metal NPs. It is the interaction of these electrons with the metal NPs that decide the photocatalytic route to be taken by each of the sample. Moreover, the photoexcited e- transfers from TiO2 to Pt until the Fermi level equilibrium is attained resulting in high conductivity, largely reducing the possibility of recombination. Now, the electrons from Fermi level of Pt NPs interact with the hydrogen ion (H+) to form hydrogen molecule (H2). In the case of Pd NPs, the metals form a surface metal oxide and when the e- is transferred from TiO2, the metal ions get reduced to metal (Pd0) in the case of Pd [66]. The electrons held by Pd are then transferred to hydrogen ion to eventually form gaseous H2 molecule. Interestingly, the involvement of ethanol is observed to act as a better electron donor [6]. During the prolonged separation of the e-/h+ pairs, the e- from the electron donors interacts irreversibly with h+ in the VB of TiO2 further separating thereby increasing the quantum efficiency. Furthermore, it suppresses the backward reaction [67].
Discussion
In order to start water splitting reaction it is need to supply some energy since it is an endothermic reaction requiring a minimum energy of 1.23eV because the Gibbs free energy change for the reaction is ΔG°=237.2kJ mol-1 or 2.46eV per molecule of H2O. This required energy coming from modified TiO2 species after its activation with certain wavelength light.
It was seen that modification of TiO2 is possible to carry out using different methods of synthesis as well as different selection of starting compounds (presence or absence of sacrificial agent) and co-catalysts. It should be emphasized that in the presence of sacrificial agents not only hydrogen is produced but also is evident simultaneous formation of CH4 and C2H6 by products. Someone should also study selectivity of the prepared photocatalysts regarding the presence of by-products. From all mention above methods of synthesis, the best yield of hydrogen is achieved with the method of nanoparticles modification of TiO2 and in the presence of water-alcohol mixtures.
Conclusion
Photocatalytic water splitting and hydrogen production as a clean way of energy can contribute to replacing fossil fuel and reduce greenhouse gas emissions. It has been studied different methods of synthesis of photocatalytic materials based on TiO2 and its use for the photocatalytic water splitting for hydrogen generation. This review paper has shown that there are advances and achievements in the development of new photocatalysts for water splitting, but more research is required especially if someone wants to scale up this process and become alternative related to the current way of hydrogen production.
Conflict of Interest
There are no conflicts to declare.
References
- EIA (2019) International Energy Outlook 2019 U.S. Energy Information Administration: Washington, DC, USA.
- Höök M, Tang X (2013) Depletion of fossil fuels and anthropogenic climate change-A review. Energy Policy 52: 797-809.
- IPCC (2018) Global Warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. IPCC, Geneva, Switzerland.
- European Commission (EC) (2020) A hydrogen strategy for a climate-neutral Europe. Brussels.
- IRENA (2019) Hydrogen: A renewable energy perspective. International Renewable Energy Agency, Abu Dhabi, United Arab Emirates.
- Ni M, Leung MKH, Leung DYC, Sumathy K (2004) Water electrolysis: a bridge between renewable resources and hydrogen. Proc Int Hydrogen Energy Forum, Beijing, China.
- Ni M, Leung MKH, Leung DYC, Sumathy K (2007) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sust Energ Rev 11(3): 401-425.
- Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358): 37-38.
- Bashiri R, Mohamed NM, Ling LY, Suhaimi NA, Shahid MU, et al. (2019) Influence of seeding layer on photoelectrochemical hydrogen production over TiO2 nanorod decorated with reduced graphene oxide. Diamond Relat Mater 94: 194-202.
- Liu Z, Lu X (2018) Multifarious function layers photoanode based on g-C3N4 for photoelectrochemical water splitting. Chin J Catal 39(9): 1527-1533.
- He H, Cao J, Guo M, Lin H, Zhang J, et al. (2019) Distinctive ternary CdS/Ni2P/g-C3N4 composite for overall water splitting: Ni2P accelerating separation of photocarriers. Appl Catal B 249: 246-256.
- Hoffmann MR, Martin ST, Choi W, Bahnemann DW, Detlef W (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95(1): 69-96.
- Lee SY, Park SJ (2013) TiO2 photocatalyst for water treatment applications. J Ind Eng Chem 19(6): 1761-1769.
- Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C Photochem Rev 1(1): 1-21.
- Khan SUM, Al-Shahry M, Ingler Jr WB (2002) Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297(5590): 2243-2245.
- Elsellami L, Dappozze F, Fessi N, Houas A, Guillard C (2018) Highly photocatalytic activity of nanocrystalline TiO2 (anatase, rutile) powders prepared from TiCl4 by sol-gel method in aqueous solutions. Process Saf Environ Protect 113: 109-121.
- Mohapatra AK, Nayak J (2018) Anatase TiO2 powder: synthesis, characterization and application for photocatalytic degradation of 3,4-dihydroxy benzoic acid. Optik 156: 268-278.
- Kernazhitsky L, Shymanovska V, Gavrilko T, Naumov V, Fedorenko L, et al. (2014) Room temperature photoluminescence of anatase and rutile TiO2 J Lumin 146: 199-204.
- Mikrut P, Kobielusz M, Macyk W (2019) Spectroelectrochemical characterization of euhedral anatase TiO2 crystals - implications for photoelectrochemical and photocatalytic properties of {001} {100} and {101} facets. Electrochim Acta 310: 256-265.
- Tahir M, Amin NS (2013) Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers Manag 76: 194-214.
- Barkhade T, Banerjee I (2019) Optical properties of Fe doped TiO2 nanocomposites synthesized by sol-gel technique. Materials Today: Proceedings 18(3): 1204-
- Li Y, Fu R, Gao M, Wang X (2019) B-N co-doped black TiO2 synthesized via magnesiothermic reduction for enhanced photocatalytic hydrogen production. International Journal of Hydrogen Energy 44(54): 28629-
- Diaz Angulo J, Arce Sarria A, Mueses M, Hernandez A, Machuca F (2019) Analysis of two dye-sensitized methods for improving the sunlight absorption of TiO2 using CPC photoreactor at pilot scale. Materials Science in Semiconductor Processing 103: 104640-104647.
- Wu T, Zhen C, Wu J, Jia C, Haider M, et al. (2019) Chlorine capped SnO2 quantum-dots modified TiO2 electron selective layer to enhance the performance of planar perovskite solar cells. Science Bulletin 64(8): 547-
- Nguyen CH, Tran ML, Van Tran T, Juang S (2020) Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Separation and Purification Technology 232: 115962-
- Wang J, Meng F, Xie W, Gao C, Zha Y, et al. (2018) TiO2/CeO2 composite catalysts: synthesis, characterization and mechanism analysis. Applied Physics A 124: 645.
- Bratovcic A (2019) Photocatalytic degradation of organic compounds in wastewaters. Technological Acta 11(2): 17-23.
- Bratovcic A (2019) Degradation of micro-and nano-plastics by photocatalytic methods. Applications Nanotechnology and Nanoscience of J 3(3): 1-9.
- Bratovcic A (2020) New solar photocatalytic technologies for water purification as support for the implementation of industry 4.0. In: Karabegović I, Kovačević A, Banjanović L, Dašić P (Eds.), Handbook of Research on Integrating Industry 4.0 in Business and Manufacturing. IGI Global, USA, pp. 385-412.
- Bratovcic A, Odobasic A, Sestan I (2017) The level of knowledge on the use of titanium dioxide as a photocatalyst in bosnia and herzegovina. American Journal of Engineering Research 6(2): 51-55.
- Bratovcic A (2020) Fotohemija i fotokataliza. In Scan d.o.o. Tuzla, Tuzla, Bosnia and Herzegovina, pp. 1-251.
- Al Azri H, Chen T, Chan A, Jovic V, Ina T, et al. (2015) The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M=Pd, Pt, Au) in different alcohol-water mixtures. Journal of Catalysis 329: 355-367.
- Kalisman P, Nakibli Y, Amirav L (2016) Perfect photon-to-hydrogen conversion efficiency. Nano Letters 16(3): 1776-
- Miseki Y, Sayama K (2019) Photocatalytic water splitting for solar hydrogen production using the carbonate effect and the z-scheme reaction. Advanced Energy Materials 9(23):
- Bratovcic A (2019) Different applications of nanomaterials and their impact on the environment. SSRG International Journal of Material Science and Engineering 5(1): 1-7.
- Bratovcic A (2020) Nanocomposite hydrogels reinforced by carbon nanotubes. International Journal of Engineering Research and Application 10(5): 30-41.
- Bratovcic A, Petrinic I (2020) Carbon based aerogels and xerogels for removing of toxic organic compounds. New Technologies, Development and Application 743-749
- Bratovcic A (2020) Synthesis, characterization, applications, and toxicity of lead oxide nanoparticles. IntechOpen.
- Bratovcic A (2020) Biosynthesis of green silver nanoparticles and its uv-vis characterization. International Journal of Innovative Science, Engineering & Technology 7(7): 170-176.
- Li H, Wu S, Hood ZD, Sun J, Hu B, et al. (2020) Atomic defects in ultra-thin mesoporous TiO2 enhance photocatalytic hydrogen evolution from water splitting. Applied Surface Science 513: 145723.
- Dosado AG, Chen WT, Chan A, Waterhouse D, Waterhouse GIN (2015) Novel Au/TiO2 photocatalysts for hydrogen production in alcohol-water mixtures based on hydrogen titanate nanotube precursors. Journal of Catalysis 330: 238-254.
- Manovah DT, Wilson P, Mahesh R, Sagayaraj P, Murugesan N, et al. (2018) Photocatalytic water splitting of TiO2 nanotubes powders prepared via rapid breakdown anodization sensitized with Pt, Pd and Ni nanoparticles. Materials Technology 33(4): 288-300.
- Escobedo S, Salvador (2013) Photocatalytic water splitting using a Modified Pt-TiO2. Kinetic modeling and hydrogen production efficiency. Electronic Thesis and Dissertation Repository.
- Kenanakis G, Katsarakis N (2014) Chemically grown TiO2 on glass with superior photocatalytic properties. J Environ Chem Eng 2(3): 1748-1755.
- Alzamani M, Shokuhfar A, Eghdam E, Mastali S (2013) Influence of catalyst on structural and morphological properties of TiO2 nanostructured films prepared by sol-gel on glass. Prog Nat Sci Mater Int 23(1): 77-84.
- Arconada N, Castro Y, Durán A (2010) Photocatalytic properties in aqueous solution of porous TiO2-anatase films prepared by sol-gel process. Appl Catal A Gen 385(1-2): 101-107.
- Anderson AL, Binions R (2014) The effect of tween® surfactants in sol-gel processing for the production of TiO2 thin films, coatings. 4(4): 796-809.
- Medina VJ, Sánchez CM, Frausto RC, Calixto S (2006) Formation of smooth and rough TiO2 thin films on fiberglass by sol-gel method. Chem Soc 50(1): 8-13.
- Pala LPR, Uday V, Gogoi D, Peela NR (2020) Surface and photocatalytic properties of TiO2 thin films prepared by non-aqueous surfactant assisted sol-gel method. J Environ Chem Eng 8(5): 104267.
- Dunuwila DD, Gagliardi CD, Berglund KA (1994) Application of controlled hydrolysis of titanium (IV) Isopropoxide to produce sol-gel-derived thin films. Chem Mater 6(9): 1556-1562.
- Wang H, Zhang L, Chen Z, Hu J, Li S, et al. (2014) Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem Soc Rev 43(15): 5234-5244.
- Iwashina K, Kudo A (2011) Rh-Doped SrTiO3 photocatalyst electrode showing cathodic photocurrent for water splitting under visible-light irradiation. J Am Chem Soc 133(34): 13272-13275.
- Kim HG, Hwang DW, Lee JS (2004) An undoped, single-phase oxide photocatalyst working under visible light. J Am Chem Soc 126(29): 8912-8913.
- Xu X, Randorn C, Efstathiou P, Irvine JTS (2012) A red metallic oxide photocatalyst. Nat Mater 11(7): 595-598.
- Maeda K, Higashi M, Siritanaratkul B, Abe R, Domen K (2011) SrNbO2N as a water-splitting photoanode with a wide visible-light absorption band. J Am Chem Soc 133(32): 12334-12337.
- Kasahara A, Nukumizu K, Hitoki G, Takata T, Kondo JN, et al. (2002) Photoreactions on LaTiO2N under visible light irradiation. J Phys Chem A 106(29): 6750-6753.
- Higashi M, Domen K, Abe R (2012) Highly stable water splitting on oxynitride TaON photoanode system under visible light irradiation. J Am Chem Soc 134(16): 6968-69671.
- Kim E, Nishimura N, Magesh G, Kim JY, Jang JW, et al. (2013) Fabrication of CaFe2O4/TaON heterojunction photoanode for photoelectrochemical water oxidation. J Am Chem Soc 135(14): 5375-5383.
- Kim H, Monllor SD, Kim W, Choi W (2015) N-doped TiO2 nanotubes coated with a thin TaOxNy layer for photoelectrochemical water splitting: Dual bulk and surface modification of photoanodes. Energy Environ Sci 8(1): 247-257.
- Hou J, Yang C, Cheng H, Jiao S, Takeda O, et al. (2014) High-performance p-Cu2O/n-TaON heterojunction nanorod photoanodes passivated with an ultrathin carbon sheath for photoelectrochemical water splitting. Energy Environ Sci 7(11): 3758-3768.
- Li Y, Xie W, Hu X, Shen G, Zhou X, et al. (2010) Comparison of dye photodegradation and its coupling with light-to-electricity conversion over TiO2 and ZnO. Langmuir 26(1): 591-597.
- Girish KS, Koteswara R (2017) Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl Surf Sci 391: 124-148.
- Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 43(22): 7520-7535.
- Idriss H (2020) The elusive photocatalytic water splitting reaction using sunlight on suspended nanoparticles: Is there a way forward? Catal Sci Technol 10(2): 304-310.
- Galinska A, Walendziewski J (2005) Photocatalytic water splitting over Pt-TiO2 in the presence of sacrificial reagents. Energ Fuel 19(3): 1143-1147.
- Sun T, Liu E, Liang X, Hu X, Fan J (2015) Enhanced hydrogen evolution from water splitting using Fe-Ni codoped and Ag deposited anatase TiO2 synthesized by solvothermal method. Appl Surf Sci 347: 696-705.
- Abe R, Sayama K, Domen K, Arakawa H (2001) A new type of water splitting system composed of two different TiO2 photocatalysts (anatase, rutile) and an IO3-/I- shuttle redox mediator. Chem Phys Lett 344(3-4): 339-344.
© 2020 Amra Bratovcic. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






