- Submissions

Full Text
Research & Development in Material Science
The Study on the Reducibility of Itakpe Iron Ore Lumps
Ocheri C1*, Njoku RE2, Urama NA3, Adams S Mohammed3, Mbah CN3, Chikezie WO4 and Ezeanyanwu J4
1,2,3Department of Metallurgical and Materials Engineering, University of Nigeria, Nigeria
3,4Department of Metallurgical and Materials Engineering, Enugu State University of Science and Technology, Nigeria
*Corresponding author: Ocheri C, Department of Metallurgical and Materials Engineering, University of Nigeria, Nsukka
Submission: May 28, 2020;Published: June 19, 2020

ISSN: 2576-8840 Volume 13 Issue 4
Abstract
The study on the reducibility on the Itakpe iron ore lumps were carried out. The chemical compositions, structural analysis and morphological structures of the ores were examined. Some analytical instruments and equipment were used to determine the Thermogravimetry (TG), the Differential Thermal Analyzer (DTA), the X-Ray Fluorescence (XRF) and X-Ray Diff action (XRD). Optical Microscopy and Scanning Electron Microscopy (SEM) with Energy Dispersive Spectroscopy (EDS) were also used for further analyses .The reducibility study was performed in the laboratories of the Department of Metallurgical and Materials Engineering, University of Nigeria, Nsukka, Nigeria. A muffle furnace with model number LABE 1210, Divine international. The heating capacity of 1500 ͦC as the heating device was used for the experiments. The samples were heated to temperature range from 800 ͦC to 1000 ͦC with temperature interval of 40 ͦC .The duration allowed for each of the experiments ranged from 20 minutes to 120minutes and also allowed interval time of 20 minutes. The iron ore lumps were taken inside six stainless steel containers with diameter 0.675cm x 0.594cm inside diameter with the mouths tightly closed with air tight covers for gas escape, the metallurgical coking coal obtained from the Ajaokuta Steel Company Limited was used as a reducing agents . The highest reduction value of Itakpe iron ore lump was obtained at 74.3% with corresponding temperature at 1000 ͦC at time rate of 120minutess. The (SEM) and (EDS) were used to determine the iron (Fe) content after the reducibility tests were performed. Some recommendations and contributions from the research work was stated to assist the would - be researchers in this field of study.
Keywords: Study; Reducibility; Itakpe; Iron ore; Lumps
Introduction
The Itakpe iron ore deposit is located northeast of Okene in the eastern part of Kogi state. This ore deposit is recently used for research works in Nigeria, This development is due to its usefulness for the operation of the Blast Furnace at an integrated steel plant in Nigeria located at the Ajaokuta Steel Company Limited. Subsequently the ores are used as feeds at the Delta Steel Company, Aladja, Delta state (Direct Reduced Iron).
The plateau is made of scattered hills, that are made of Precambrian gneisses and granites that are surrounded with about 200m to 300m. The ore deposit is estimated to have reserve of over 300 million tones. The verified reseve has been put to be around 200 million tones [1].
Reducibility
This is known as the velocity of iron oxide transformation to metal by effect of reduction gas, it is also known as a time needed for a complete iron oxide reduction. The value of the reduction rate is the metallic charge weight loss per a time unit. The weight loss caused by the charge oxygen transformation into gas.
It has a lot of influence on the blast furnace operation that is very essential it can serves as fuel consumption for the determination selection and completeness of utilizing of the lumpiness. The reducibility test method is one of most important methods to determine the behavior of natural and processed iron ores. Iron ores are upgraded to higher iron ore content through beneficiation processes.
Research Methodology
Materials
Itakpe iron ore: The topography of the region is a plateau rising gently to the north-east of Okene in the eastern part of Kogi State, down to the river Niger. The plateau is bestrewn with scattered hills, which are made of Precambrian gneisses, and granites that overlook the surrounding by about 200m to 300m [1]. The Itakpe iron ore specimen is known to be a compacted, crystalline like banded iron ore which has various colours like dark grey, brown and black. The Itakpe iron ore slightly magnetic in nature.
Table 1: Shows the chemical compositions of Itakpe Iron Ore using XRF Method.
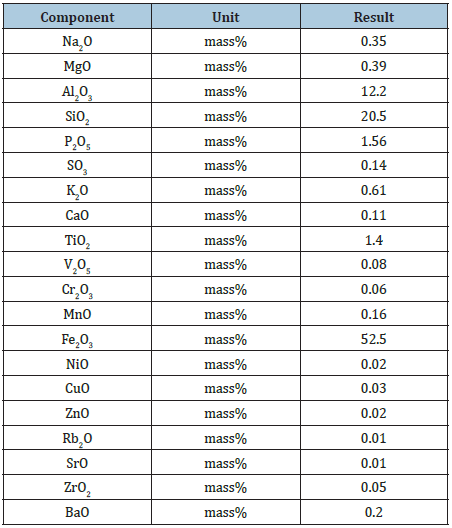
Chemical analysis of Itakpe: Table 1 shows the analytical result the Itakpe iron ore chemical compositions. This was determed with the use of the X- Ray Fluorescence (XRF) methods. The experiment was performed at the laboratory of Tshwane University of Technology, South Africa [2].
X-Ray Diffraction (XRD) on Itakpe iron ore:
Figure 1:
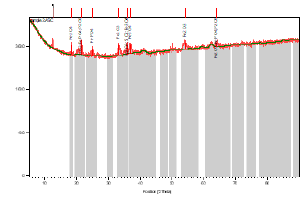
Figure 1 & Table 2
Table 2:Identified patterns list: Itakpe iron ore.
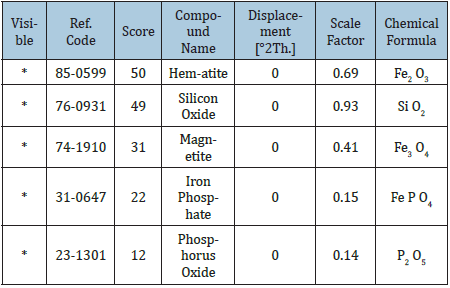
Source from the XRD Analysis
Metallurgical coking coal: The coking coal used for this experiment was selected from among the imported Metallurgical Coking Coal at the Ajaokuta Steel Company Limited. The chemical compositions of the coking coal was determined by the supplier of the coking coal to the steel plant before importing them to the steel company. The chemical compositions of the coal are shown in the Table 3 below:
Table 3: Chemical analysis of metallurgical coking coal.

Source: Ajaokuta Steel Company Limited, Ajaokuta
Equipment and methods
Some important equipment that is relevant to the study of reducibility was used for this research work.
Sample handler: The Itakpe iron ore lumps were carefully packaged to prevent them from breaking.
Muffle furnace: A Muffle furnace was used for the heating of the iron ores lumps to the required temperatures and time. The Muffle Furnace used for this experiment has a model number LABE 1210, Divine international with heating capacity of 1500 ͦC.
Inverted metallurgical microscope: This instrument with model name XJL-17 was used for the examination of the microstructures of the iron ore lump samples before and after the reducibility tests.
Methods
Tables 1 Shows the chemical compositions of the Itakpe Iron ore lumps. The iron ore concentrate has phosphorus in traces The morphologies of the ore was determined using the X-Ray Diffraction methods as shown in Figure 2 with other details in Table 2.
Figure 2: Shows the microscope of the Itakpe iron ore before the reducibility experiment.

Experimental procedure of reducibility: Reducibility is a summary of raw materials properties, which determines the rate of conversion of iron oxides to metals by treatment with reluctant. A measure of reducibility is represented by a weight loss of ore samples per time unit caused by transition of oxygen into gas [3].
Experimental procedure for reduction studies
- The Itakpe Iron ore lumps were used for these experiments, while a metallurgical coking coal obtained from the Ajaokuta Steel Company Limited was also used as reducing agent (reduant).
- The collected iron ores lumps were crushed to 15-20mm sizes
- The collected metallurgical coking coal was crushed to -5+15 size.
- The chemical compositions of the Metallurgical coking coal is shown in Table 3
- The crushed iron ore lumps were dried in the laboratory dry oven to eliminate moisture content that was present in the ores as they were subjected to a temperature of 120 ͦC.
- The crushed iron ore lumps were taken inside six stainless steel containers (size: 60.75mm height × 59.40mm inside diameter) with mouths tightly closed by air tight covers having out let for exit gases. Then the lumps were surrounded with metallurgical coking coal, which serves as reducing agent in the experiments at various period and time.
The prepared samples were placed at the centre of the crushed coking coal. The processes were to ensure that the reducing processes were performed without any hindrance. The muffle furnace was used to heat up the iron ore lumps to the required temperatures of 800 ͦC, 840 ͦC, 880 ͦC, 920 ͦC, 960 ͦC and 1000 ͦC, at 8 ͦC per minute’s rate. The samples got soaked at various temperatures as indicated above by varying the soaking period in the range of 20-120 minutes.
The reducibility tests were performed in accordance at the stipulated temperatures. Each of the containers was properly labeled for the specific experiments. The samples were brought out from the muffle furnace at designated interval of 20 minutes and the same trend were followed for the rest samples at an interval of 40 minutes up to 120 minutes of residence in the furnace. The containers containing the samples were brought out from the muffle furnace and were allowed to cool at the normal atmospheric air. The weight losses analyses of the iron ore lumps, were determined, and recorded. The reducibility of the iron ore lumps were calculated by using the following formula.
Microscope examination
Figure 3: Reduced ore @800 oC (Partially reduced) after reducibility experiment.
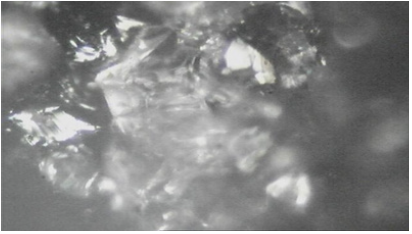
Figure 4: Reduced ore @840 oC (Partially reduced) after reducibility experiment.

Figure 5: Ore reduced @880 oC (Partially Reduced) reducibility experiments.
The Inverted Metallurgical Microscope was used to examine the iron ore lumps. Some important distinct phases were identified in the Itakpe iron ore with the use of optical microscopy; the iron ore shows some grey like structures, some whitish mottled and blackish / whitish location. These structures and features of the Itakpe iron ore lumps were observed using inverted Metallurgical Microscope as shown in Figure 2 before the reducibility tests were carried out (Figure 3-8).
Figure 6: Ore reduced @920 oC (Partially reduced) after reducibility experiment.
Figure 7: Ore reduced @ 960 oC (Fully (Reduced) after reducibility experiment.

Figure 8: Ore reduced @ 1000 oC (Fully reduced) after reducibility experiment.
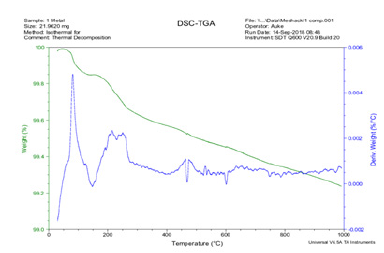
The Thermogravimetric Analysis (TGA) of Itakpe iron ore
The line with blue colour runs on 30 ͦC and moved upwards until it got to the peak value of Deriv weight (%C) of 0.0048. This value declined and rose until it achieved a stable value and finally attained a value of 0.0007at the Drevi weight (%C). On the other hand, the line with light green colour indicates weight (%) versus temperature. The weight (%) started from 100 and continues to decline until it got to 1000 ͦC with corresponding value at 99.2. Figure 9 shows the Isothermal behavior of the thermal decomposition of the Itakpe iron ore.
Figure 9: Show the Thermogravimetry Analysis (TGA) performed on Itakpe iron ore.
Scanning Electron Microscope (SEM) of Itakpe iron ore
Examination by Scanning Electron Microscopy (SEM) /Energy-Dispersive Spectroscopy (EDS) shows that there are grey phase with quartz, the white phase hematite, and the mottled areas intergrowths of hematite and magnetite (Figure 10).
Figure 10: Shows the Scanning Electron Microscopy SEM of the Itakpe Iron ore at 100μm.

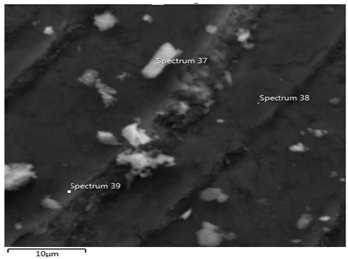
Electron image of the spectrum location 37, 38 and 39 at 10µm Itakpe iron ore: Figure 11 shows the three-spectrum location that was taken in order to determine the concentration of ore. The locations of the spectrum are 57 38 and 39 at 10µ
Figure 11: Shows the Scanning Electron Microscopy SEM of the Itakpe Iron ore at 10μm.
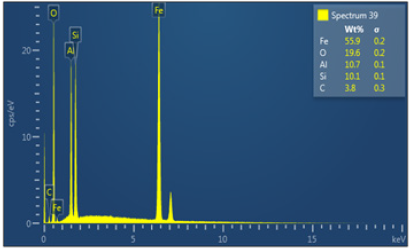
Energy-Dispersive Spectroscopy (EDS) of Itakpe iron ore : Figure 12 shows spectrum 39 where the concentration of the elements are distributed with weight (%) concentration ranging from Fe, O Al, Si and C and with their corresponding density values.
Figure 12: Shows energy dispersive spectroscopy.
Results and Discussion
Results
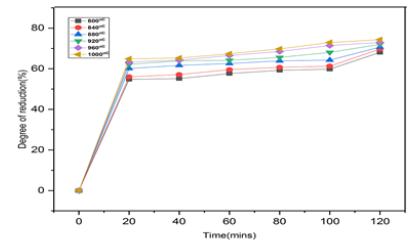
Table 4 & Figures 13 shows the reducibility as a function of furnace holding time for the reduction of Itakpe iron ore lumps at temperature between 800 ͦC-1000 ͦC.
Table 4: Itakpe iron ore lumps.
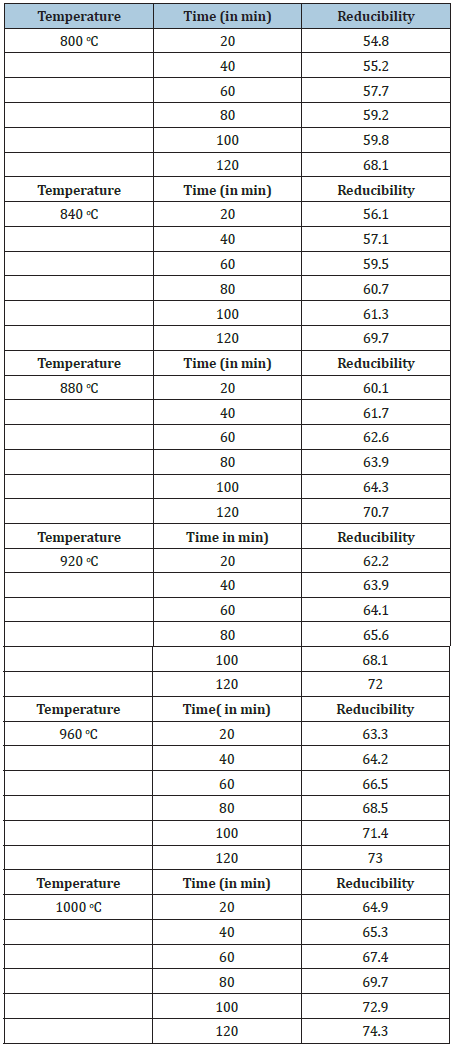
Figure 13: Degree of reduction (%) of Itakpe iron ore lumps Versus Time (in mins) at temperature between 800 oC to 1000 oC.
Discussion
The study of the reducibility on the Itakpe iron ore lumps:
Reducibility iron ore lumps
The samples were heated up to various temperatures ranging from 800 ͦC-1000 ͦC and the samples were allowed to soak for period of time ranging from 20 minutes to 120 minutes. The corresponding values collected were recorded and these obtained values were used to plot graphs as function of furnace holding time for the reduction of the ore at temperature between 800 ͦC-1000 ͦC.
The ores cracked into fine fragments during reduction at 960 ͦC for 80 minutes to 1000 ͦC for 120minutes. These results could be found from Figure 14-25. Figure 19 shows that the ore lumps reduced @960 ͦC for 120 minutes with significant fragmentations and Figure 25 showed that the Itakpe iron ore reduced @ 1000 ͦC for 120 minutes total fragmentation of fine iron ore lump particles .
The analyses on the ores are attributed to the higher rate of Fe2O3-Fe3O4 transformation and generation of higher thermal strain. An increased degree of Fe2O3-Fe3O4 transformation increases the volumetric strain and thus the cracking tendency.
Figure 14: Shows the reduced Ore @ 960 oC for 20mins.

Figure 15: Shows the reduced Ore @ 960 oC for 40mins.

Figure 16: Shows the reduced Ore @ 960 oC for 60mins.

Figure 17: Shows the reduced Ore @ 960 oC for 80mins.

Effect of time on degree of reduction
The research work carried out indicated that the rapid heating of the ore lumps have significant effects on the heating time. The reduction of the ore behaviour showed some significant effects on the reducibility tests. It was discovered that all the reduced lumps reduced completely within 120 minutes. The excessively high reducibility in the first 40 minutes was generally because of the release of volatiles from coal used as a result of their reformation into H2, CO, etc.
Figure 18: Shows the reduced Ore @ 960 oC for 100mins.

Figure 19: Shows the reduced Ore @ 960 oC for 120mins.

Figure 20: Shows the reduced ore @ 1000 oC for 20mins.

The major participation of these reducing gases in the reduction of iron oxide (i.e. an appreciable presence of H2 and CO in the reduction chamber gives a boost in the reduction rate). The decrease in reduction rate with increasing time above 60 minutes was because of the combined effects of an increase in product metallic layer thickness and diminished evolution of volatile matter from the coal. An increase in the thickness of iron layer products offer greater resistance in the diffusion of carbon and reducing gas to the surface of unreduced iron oxide.
Effect of heating mode on the reducibility tests
Figure 21: Shows the reduced ore @ 1000 oC for 40mins.

Figure 22: Shows the reduced ore @ 1000 oC for 60mins.

Figure 23: Shows the reduced ore @ 1000 oC for 60mins.

Figure 24: Shows the reduced Ore @ 1000 oC for 100mins.

Figure 25: Shows the reduced Ore @ 1000 oC for 120mins.

The effect of heating mode on the samples was studied with a view to determine its effects on the reducibility of the ores. The ore were reduced by using coking coal at temperature range between 800 ͦC-1000 ͦC (the soaking times were varied from 20 minutes -120 minutes at an interval of 20 minutes). These experiments were performed under rapid and slow heating conditions.
It was noted that rapid heating have slowing heating effects on reduction temperature which gives appreciably higher degree of reduction. It was more likely that rapid heating from 920 ͦC to 1000 ͦC causes higher rate of volatile matter escaping from the metallurgical coking coal, thereby providing less time for H2 and CO (reducing gases) on the ores. The results indicate that there were higher rate of reducibility during the rapid heating ore at lower heating operation. Volatile matters released from the coal were done at a slower rate.
Scanning Electron Microscopy (SEM) of ore (reduced ore at 800 ͦC, 920 ͦC and 1000 ͦC)
The ore @800 ͦC for 120 minutes, 920 ͦC for 120 minutes and 1000 ͦC for 120 minutes after the reducibility tests were performed : SEM examinations show the morphology of the sample at 100µm (Figure 26-28).
Energy-Dispersive Spectroscopy (EDS) of ore after the reducibility tests on samples at 800 ͦC, 920 ͦC and 1000 ͦC
Figure 29a & 29b show ore at 800 ͦC for 120minutes after the reducibility test was carried out. The concentrations of the elements are distributed in weight (%) concentration ranging from O, Fe, C, Al, Si and P and with their corresponding density values.
Figure 26: Itakpe iron ore @ 800 oC (SEM) 100μm.

Figure 27: Itakpe iron ore @ 920 oC (SEM) 100μm.
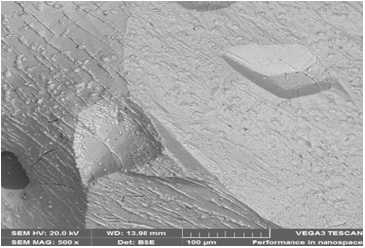
Figure 28: Itakpe iron ore @ 1000 oC (SEM) 100μm.
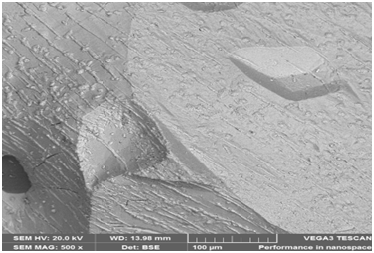
Figure 29a: Shows electron image 4 of the Itakpe Iron Ore @ 800 oC after reducibility test.
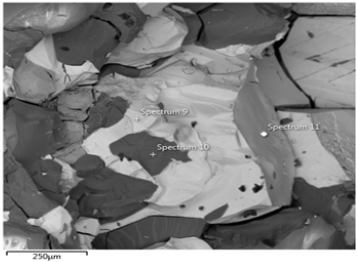
Figure 29b: Shows the EDS of Itakpe iron Ore @ 800 oC after reducibility test.

Figure 30a: Shows electron image 4 of the Itakpe Iron Ore @ 920 oC after reducibility test.
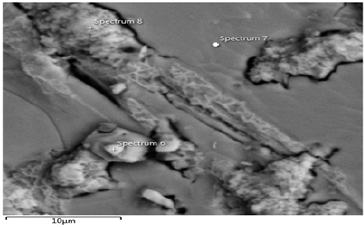
Figure 30b Shows the EDS of Itakpe iron Ore @ 920 oC after reducibility test.
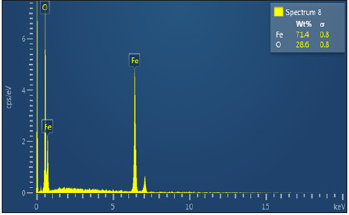
Figure 31a: Shows electron image 4 of the Itakpe Iron Ore @ 920 oC after reducibility test.
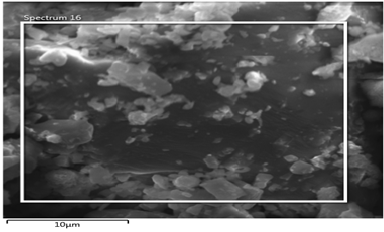
Figure 30a & 30b show the ore at 920 ͦC for 120 minutes after the reducibility test was carried out. The concentration of the elements is distributed with weight (%) concentration on Fe and O with their corresponding density values
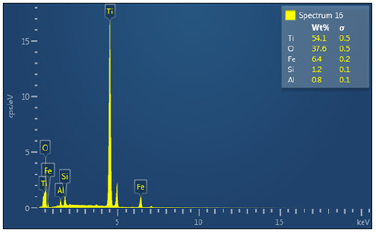
Figure 31a & 31b show the ore at 1000 ͦC for 120 minutes after the reducibility test was carried out. The concentration of the elements are distributed with weight (%) concentration ranging from Ti, O, Fe, Al, and Si with their corresponding density values.
Figure 31b: Shows the EDS of Itakpe iron Ore @ 920 oC after reducibility test.
Summary of the analysis on results after the reducibility tests (SEM and EDS)
The results on Table 5 indicates that the ore reduced at 800 ͦC for 120 minutes and had a corresponding value of iron (Fe) content of 84.5 wt per cent , the Fe content reduced from 84.5 wt per cent to 71.4 wt per cent when the samples were subjected to a heating temperature of 920 ͦC for 120 minutes . At 1000 ͦC for 120 minutes , the Fe content reduced to 6.4 wt per cent. The analyses translate to mean ores fully reduced at this temperature and time .This can be further showed that the ores reduced in this order 800 ͦC>920 ͦC>1000 ͦC . The results obtained were also represented on a graph showed in Figure 32.
Conclusion
The study of the reducibility ores were intensively carried out. The studies carried out revealed the followings:
- The time of reduction: The ores indicate that there was greater influence on the level of reducibility. It was observed that reducibility increased with increase with time.
- The reduced ores are identical in all the studies. The use of the metallurgical coking coal as reducing agent had great effects on the lumps as there were significant influences on the reducibility on the tested samples.
From the findings of the study, the results and data obtained could be used for further studies .The rest ore deposits in the country could also be subjected to some experimental investigations and processes. These processes investigated and subjected to the reducibility test in order to generate t necessary data for future use.
Table 5: analysis of results of samples after performing the reducibility tests using SEM and EDS on Itakpe iron ore.
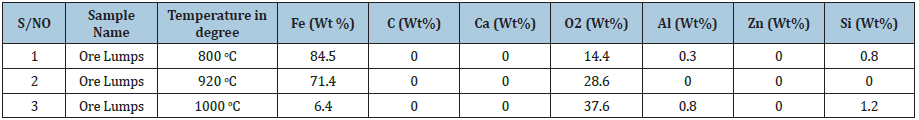
Figure 32: Shows the Fe content of Itakpe iron ore after the reducibility test @ 800 oC, 920 oC and 1000 oC using SEM and EDS.

Recommendations for Future Research
- A lot of resources is needed to perform this type of research work due to its capital involvement. In respect to this, some of the research institutions, universities and Industries could provide enabling environment for students and researchers perform their investigations / studies. In some cases waivers should be granted and sometime cost should be reduced.
- It was discovered that ample time is required to complete this type of research work.
- Raw materials sourcing are also a very high challenge and the cost of obtaining them is sometime very outrageous.
- The accessibility to source for the raw materials was very difficult as a lot of money will be required to obtain them. The security network of the ore locations and the terrain are not easily accessible. Efforts should therefore be made by all the stake holders to always assist researchers, students and other individuals by creating enabling opportunities for them to access to these raw materials.
- As regards the equipment used in these experiments, some of them were not readily available in Nigeria: such as like SEM with EDS, TA, DTA), XRF, XRD and other facilities. These issues posed lots of difficult as the samples had to be sent to South Africa.
- The collection of the test results are dependent on when the results were ready as this in most case takes a long time couple with the cost of performing such tests. Even when the equipments are available in Ngeria!, they are most cases bedeviled with issues ranging from faulty parts, total breakdown , in accuracy , doubts results and non compliance with international standard or global best practices.
- The cost of performing such experiments are relatively too high for an average students/ researchers to afford, therefore, Governments and other research Institutions, Universities and Industries should assist in support the researcher in order to encourage them.
- In view of the above therefore, the Universities, Research Institutes in Nigeria should collaborate with agencies like the NLNG, TETFUNDS, PTDF, SHELL PB CHEVRON and AGIP etc to supply equipments that will aid and facilitate research work. This with a view to collaborate with students/researchers in order to achieve optimal results.
- The research work performed on the ore is not exhaustive as more work could be carried out with other iron ores available within Kogi State like the Agbajanoko, Oshokosho, Konto Karfi, BassaNge and Muro and in other States of the Federation Nigeria.
References
- Adeleye RA (1964) The occurrence of Oolitic iron ore in the middle niger basin in geology of Nigeria”. In: Kogbe CA (Ed.), Edition Elizabeth press, Lagos, Nigeria, pp. 1-10.
- Akande JM, Adebimpe RA (2004) Physical and chemical analysis of Itakpe iron ore waste. Nigeria Journal of Engineering Research and Development 3(2): 10-15.
- Mohammed RA, Dada ET, Hassan SB, Abdulwahab M (2014) Assessment of mechanical properties of pellets produced from Itakpe/Agbaja iron ore blends. A paper published in the Nigerian Metallurgical Proceeding at the 30th Annual Conference and Annual General Meeting of the Nigerian Metallurgical Society at the Raw Materials Research and Development.
© 2020 Ocheri C. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






