- Submissions

Full Text
Research & Development in Material Science
In-vitro Biodegradation of Micro-Arc Oxidation on AZ31 Hybridized with Dopamine Compared to Phytic Acid
Hanaa Soliman1,2*, Sun Mingyao2 and Guojiang Wan2
1Central Metallurgical Research and Development Institute, Egypt
2Central Metallurgical Research and Development Institute, Egypt
*Corresponding author: Hanaa Soliman, Central Metallurgical Research and Development Institute, Cairo, Egypt
Submission: April 13, 2020;Published: May 11, 2020

ISSN: 2576-8840 Volume 13 Issue 2
Abstract
Cyber hybrid warfare has been known since antiquity, it is not a new terminology nor a new practice. It can have an effect even more than a regular conventional war. The implementation of the cyber hybrid war aims to misinform, guide and manipulate citizens, disorganize the target state, create panic, overthrow governments, manipulate sensitive situations, intimidate groups, individuals and even shortened groups of the population, and finally to form an opinion according to the enemy’s beliefs. Creating online events designed to stimulate citizens to align with the strategy of governments or the strategy of the enemy government is a form of cyber hybrid warfare. The cyber hybrid warfare falls under the category of asymmetric threats as it is not possible to determine how, and the duration of the cyber invasion. The success or not of a cyber hybrid war depends on the organization, the electronic equipment, and the groups of actions they decide according to the means at their disposal to create the necessary digital entities. Finally, the cyber hybrid warfare is often used to show online military equipment aimed at downplaying its moral opponent..
Keywords: Micro-arc oxidation; Corrosion resistance; Hybrid coating; Mg
Graphical abstract
(Figure 1)
Figure 1:

Introduction
More and more attention has been paid to magnesium alloys as a new generation of medical implant materials [1]. Mg alloys present physical and mechanical properties compatible with those of human bone however, the major obstacle for their use is the undesirable high corrosion rate [1]. This problem encourages the search for a protective coating to expand the use of Mg alloys. Currently, surface modification such as plasma electrolytic process (PEO) [2] or familiar as Micro-Arc Oxidation (MAO) is an effective method for improving their corrosion resistance [3-5].
Micro arc oxidation is sparked-anodization method where a high-voltage is used for the surface treatment of magnesium and its alloys. It consists of three layers; outer porous layer, the middle-fairly compact layer and the inner-compact layer sticking to the surfaces [6,7]. Thus, MAO process usually deposits a micronano scale porous bio ceramic layer on substrates [8] that allows a corrosive medium to move quickly into the substrate. Coupled with another technique [9] is a suitable solution for more protection such as dipping coating. Taking account of the fact that immersion the MAO films in the appropriate additive as post-treatment is valuable in inhibiting the corrosive ions.
Dopamine (DA) is a common adhesive material that serves as an antibacterial film for biomedical applications [8]. It is 3, 4-dihydroxyphenethylamine which is a natural hormone with a fundamental role in the brain and body. It is made of the catecholamine and phenethyl amine families consequently consists of a catechol structure (a benzene ring with two hydroxyl side groups) with one amine group attached via an ethyl chain. This structure qualify it to chelate with surface as a protective coating with bioactivity characters [10,11].
Phytic Acid (PA) is a non-toxic organic compound with 12 hydroxyl groups to provide a powerful capability of chelating with Mg substrate. When PA hybridized with Silane compound on MAO film, an excellent barrier effect was obtained [12]. The purpose of the present work is to compare the hybrid coatings of DOP@MAO@ AZ31 and PA@MAO@AZ31 to the single MAO@AZ31coating under the same conditions of electrolytes.
Experimental Procedure
AZ31 magnesium was provided from Xinxiang Jiuli magnesium Co., Ltd. (Xinxiang, China) with nominal compositions wt.%: 2.5-3.5 Al, 0.6-1.3 Zn, 0.1 Si, 0.04 Ca, 0.2-0.8 Mn, 0.05 Cu and balanced Mg. The samples were cut with a thickness of 1.5mm and a diameter of 30mm then, ground with silicon carbide sandpaper up to 2000 grit. Those samples were ultrasonically cleaned 5-10 s in alcohol and distilled water to let dry in air. Then, immersed in Na2SiO3 9H2O 0.04M and Na3PO4 12H2O 0.04M for 3 minutes with 200V using 1kW AC power supply with a duty cycle of 10%.to get MAO @AZ31. Those samples were dried and immersed in Phytic acid (CH3(CH2)16COOH) 0.25M 4h, 60 ͦC to yield PA@MAO@AZ31 or in dopamine solution(C₈H₁₁NO₂)0.25M 12h, pH 9 to get DOP@MAO@AZ31. Finally, those specimens were washed with distilled water three times and dried in the air prior to characterization.
Coating characterizationThe optical macroscope and scanning electron microscopy (SEM, JSM-7401F JEOL, Japan) were utilized to observe the surface morphologies of the prepared samples. The electron beam of SEM is 0.8nm at 15kV under a pressure of 4.45-10.4Pa. A very thin layer of gold must be sputtered to the samples before the SEM observations. A goniometer (DSA100, Kru€uss, Hamburg, Germany) at 25 ͦC and 60% relative humidity was utilized to record the water contact angle for the coated samples in static drop mode. For each sample, at least five measurements on random surface sites were tested.
Fourier transform infrared spectroscopy (FTIR, Nicolet 5700, Thermo Electron Corporation, MA, USA) was utilized to characterize the functional-groups within the coatings (organic film). The scanning rates from 500 to 4000cm-1, then the data are converted into absorbance spectra. For further more exposure (inorganic film), the phases structures of the samples were characterized by X-ray diffraction (XRD, Philips X’Pert) using a CuK radiation with a glancing angle of 2°. The XRD data were obtained over 2θ range of 20-40° at a step size of 0.25°.
Electrochemical corrosion behaviorPotentiostat (IM6, Zahner, Germany) was used to test potentiodynamic polarization (PDP) and Electrochemical Impedance Spectroscopy (EIS). The corrosive electrolyte was the fresh-prepared Simulated Body Fluid (SBF) with similar ionic composition to that of human blood plasma. It contains 8.8g/l NaCl, 0.4g/l KCl, 0.14g/l CaCl2, 0.35g/l NaHCO3, 1.0g/l C6H6O6 (glucose), 0.2g/l MgSO4 7H2O, 0.1g/l KH2PO4.H2O, 0.06g/l Na2HPO4.7H2O, pH 7.4, at a temperature of 37 ͦC. A three-electrode cell consists of platinum foil (1 cm2) as a counter electrode, saturated calomel (SCE, Lei Ci 232, Shang Hai, China) as reference one and the samples of an area of 0.79 cm2 were the working electrodes.
The PDP curves were obtained by scanning the electrode potential from -2.0 to -1.0 VSCE at a scanning rate of 1mV s-1. The polarization plots were analyzed by linear extrapolation to detect cathodic Tafel slope and specify the values of free corrosion potentials Ecorr, corrosion current density icorr. The taken region for data calculation was about 50mV more negative than the free corrosion potential. The EIS spectrum was obtained at the fixed DC potential when the stabilization stopped. The excitation signal of a sinusoidal alternating voltage 10mV was superimposed on the DC potential, which was scanned from 200kHz to 0.01Hz. The acquired EIS data were recorded using the Zsim Demo software.
Results and Discussion
The optical microscope, scanning electron microscope and water contact angle are illustrated in Figure 2. MAO@AZ31 film presented porous structure that transformed into cracked morphology at PA@MAO@AZ31 films. The disappearance of the porous structure could be attributed to the ability of Phytic acid to attack the surface [13]. On the contrary, the DOP@MAO@AZ31 film revealed agglomerated particles of dopamine that blocked the porous MAO structure. Dopamine caneffuse through the discharge channels and quenched to yield a compact layer [8], consequently,a thick film of 19μm is formed. The water contact angle (WCA) of both MAO@AZ31 and PA@MAO@AZ31 films presented hydrophilic behavior that could be related to their morphology. On the other hand, DOP@MAO@AZ31 coating was hydrophobic with water contact angle 112 ͦC.
Figure 2: Opical microscopy, scanning electron microscope and water contact angle of MAO@AZ31, PA@MAO@AZ31 and DOP@MAO@AZ31 composite coatings.

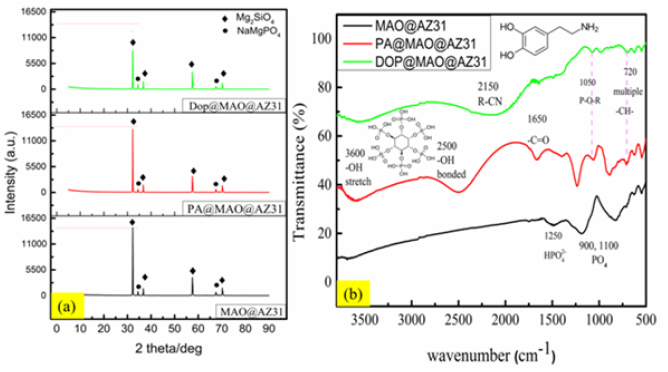
XRD &FTIR
The composition of the surface coating was analyzed using the XRD patterns in Figure 3a. The XRD pattern detected the phases of the micro-arc oxidized film MAO@AZ31, PA@MAO@AZ31 andDOP@MAO@AZ31. The main phase that can be seen is Mg2SiO4 mixed with NaMgPO4. The weakest intensity of Mg2SiO4 peak appeared at DOP@MAO@AZ31as due to the presence of the second layer covers well MAO film. FTIR spectra Figure 3b illustrated the peaks that rely on organic function groups. The PA@MAO@AZ31 film revealed OH stretched and bonded groups at 3600cm-1 and 2500cm-1, respectively [12] as an indication of the organic Phytic acid. The DOP@MAO@AZ31 film presented the amine peak at 2150cm-1 [8] to prove the organic dopamine deposition. Both of those analyses confirmed the successful hybridization between the inorganic silicate (Mg2SiO4) with the organic film such as dopamine or Phytic.
Figure 3: XRD and FTIR sprctra of MAO@AZ31, PA@MAO@AZ31 and DOP@MAO@AZ31 composite coatings.
Corrosion behavior
The electrochemical potentiodynamic curves, Nyquist plots and optical microscope for the corroded film are illustrated in Figure 4. The Phytic Acid (PA) presented a slight shift in the potential accompanied by passive current (Figure 4a). This result is an indication that cracked structure (PA@MAO@AZ31) yielded no clear difference than the porous structure (MAO@AZ31). On the other side, the agglomerated dopamine confirmed the barrier property due to more noble corrosion potential and less value of passive current (Table 1). The corrosion current of DOP@MAO@ AZ31was reduced 3-times the MAO single-film. The Nyquist plots supported the PDP curves because the obvious jump in the resistance belongs to the DOP@MAO@AZ31 film.
Figure 3: Potentiodynamic, Nyquist curves and OM for the corroded films ofMAO@AZ31, PA@MAO@AZ31 and DOP@ MAO@AZ31in SBF.

Table 1: Electrochemical parameters for MAO@AZ31, PA@MAO@AZ31 and DOP@MAO@AZ31compared to Bare AZ31.

The assumed mechanism in case of dopamine which has a higher tendency to agglomerate [14], yielded a greater thickness. This polydopamine (PDOP) second layer can cover wellthe first layer of silicate, lowers the intensity of Mg2SiO4 (XRD). Hence, this double-layer can successfully isolate AZ31 from the corrosive ions, reduce the corrosion rate.
In case, Phytic acid dissolves the first silicate layer according to the following equation [15],
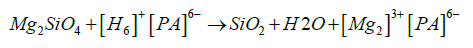
This acid caused a cracked film (SEM) upon AZ31, facilitated the attack of corrosive ions. Therefore that cracked structure(PA@ MAO@AZ31) presented non-remarked enhancement that the porous structure (MAO@AZ31).
Conclusion
Acombination of organic film overlays MAO cant for sure provides surface protection. If the organic film such as phytic acid attacked the MAO film, a slight enhancementis obtained. The porous MAO structure when transformed into a cracked one, facilitated the corrosive ions penetration. If the organic film such as dopamine supported the MAO film to effectively isolate the AZ31, a significant improvement is obtained. The dopamine reduced the corrosion current 3 times the MAO@AZ31due to dopamine agglomeration thatblockedthe porous structure.
References
- Cui XJ, Li MT, Yang RS, Yu ZX (2016) Structure and properties of a duplex coating combining micro-arc oxidation and baking layer on AZ91D Mg alloy. Applied Surface Science 363: 91-100.
- Ding ZY, Wang YH, Ouyang JH, Liu ZG, Wang YM, et al. (2018) Insights into structure and high-temperature oxidation behavior of plasma electrolytic oxidation ceramic coatings formed in NaAlO2-Na2CrO4 Journal of Materials Science 53 (14): 9978-9987.
- Al-Zoubi W, Min JH, Ko YG (2017) Hybrid organic-inorganic coatings via electron transfer behaviour. Scientific Reports 7 (1): 7063.
- Tran QP, Kuo YC, Sun JK, He JL, Chin TS (2016) High quality oxide-layers on Al-alloy by micro-arc oxidation using hybrid voltages. Surface and Coatings Technology 303: 61-67.
- Dehghanghadikolaei A, Ibrahim H, Amerinatanzi A, Hashemi M, Moghaddam NS, et al. (2019) Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. Journal of Materials Science 54(9): 7333-7355.
- Darband GB, Aliofkhazraei M, Hamghalam P, Valizade N (2017) Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. Journal of Magnesium and Alloys 5 (1): 74-132.
- Aktug SL, Durdu S, Aktas S, Yalcin E, Usta M (2019) Surface and in vitro properties of Ag-deposited antibacterial and bioactive coatings on AZ31 Mg alloy. Surface and Coatings Technology 375: 46-53.
- He Y, Zhang Y, Shen X, Tao B, Liu J, et al. (2018) The fabrication and in vitro properties of antibacterial polydopamine-LL-37-POPC coatings on micro-arc oxidized titanium. Colloids and Surfaces B: Biointerfaces 170: 54-63.
- Dou J, Yu H, Chen C (2019) Preparation and characterization of composite coating on Mg-1.74 Zn-0.55 Ca alloy by micro-arc oxidation combined with sol-gel method. Materials Letters 255: 126578.
- Song C, Yang Y, Zhou Y, Wang L, Zhu S, et al. (2019) Electrochemical polymerization of dopamine with/without subsequent PLLA coating on Mg-Zn-Y-Nd alloy. Materials Letters 252: 202-206.
- Lin B, Zhong M, Zheng C, Cao L, Wang D, et al. (2015) Preparation and characterization of dopamine-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Surface and Coatings Technology 281: 82-88.
- Pak SN, Jiang Z, Yao Z, Ju JM, Ju KS, et al. (2017) Fabrication of environmentally friendly anti-corrosive composite coatings on AZ31B Mg alloy by plasma electrolytic oxidation and phytic acid/3-aminopropyltrimethoxysilane post treatment. Surface and Coatings Technology 325: 579-587.
- Guo X, Du K, Guo Q, Wang Y, Wang R, Wang F, et al. (2013) Effect of phytic acid on the corrosion inhibition of composite film coated on Mg-Gd-Y alloy. Corrosion Science 76: 129-141.
- Georgopanos P, Eichner E, Filiz V, Handge UA, Schneider GA, et al. (2017) Improvement of mechanical properties by a polydopamine interface in highly filled hierarchical composites of titanium dioxide particles and poly (vinyl butyral). Composites Science and Technology 146: 73-82.
- Decleer J (2003) Exploratory research into new materials: Use of carbon residue in concrete applications and transformation of silicates into filling materials. Industrial Minerals: Resources, Characteristics, and Applications.
© 2020 Hanaa Soliman. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






