- Submissions

Full Text
Research & Development in Material Science
Titanium Oxide and their Effects on Structure, Physical Properties and Electrochemical Corrosion Parameters of Alloys
Abu Bakr El- Bediwi1*, Eman Kashita2, Feryal Dawood3 and Fathia Khalifa4
1Metal Physics Lab, Physics Department, Faculty of Science, Mansoura University, Mansoura, Egypt
2Educational Services, Qassim University, Ministry of High Education, Kingdom of Saudi Arabia
3Basic education college, University of Diayala, Iraq
4Sirte University, Sirte, Libya
*Corresponding author: Abu Bakr El- Bediwi, Physics Department, Faculty of Science, Mansoura University, Mansoura, Egypt
Submission: November 03, 2019;Published: November 12, 2019

ISSN: 2576-8840 Volume 12 Issue 2
Abstract
Effect of adding titanium oxide nanoparticles to bismuth or tin based alloys on microstructure, physical properties and corrosion parameters has been studied. Surface properties of tin or bismuth alloys such as hardness and corrosion parameters improved after adding titanium oxide. Matrix structure of tin or bismuth alloys changed after adding titanium oxide due to form a solid solution with caused a lattice distortion or growth formed phases. A little variation caused in melting temperature for bismuth or tin based after adding different ratio from titanium oxide.
Keywords: Alloys, Titanium oxide, Corrosion parameters, Microstructure, Mechanical properties
Introduction
Materials are so significant in the development of civilization. Almost no metals are used in pure form, but they are always combined with each other to recover one or more properties. An alloy may be defined as a substance that has the metallic properties and is composed of two or more chemical elements of which at least one is a metal. Most alloys are great importance in industry and in the arts than are the pure metals. Several researches reported that, modification structure by adding alloying elements to tin or bismuth-based alloys caused minor/ or major effects on measured physical properties such as elastic modulus, hardness, meting temperature, internal friction, spreading, resistivity and electrochemical corrosion behavior with corrosion parameters [1-17]. Microstructure, mechanical and thermal properties of bismuth or tin based alloys are studied [18-23] studied at different conditions. Matrix structure of these alloys is changed which effects on all measured physical properties.
Titanium dioxide (TiO2)Titanium and some of its alloys are used as biomaterials for dental and orthopedic applications. Titanium is used in condensers and turbine blades in electric power plants. It is also incorporated into the architecture of buildings, roofs, piping and cable. Titanium dioxide has a wide range of applications, from paint to sunscreen to food coloring. It has eight modifications rutile, anatase, brookite, α-PbO2-like, baddeleyite like, cotunnite -like, orthorhombic OI and cubic phases) also exist. Titanium Dioxide has high gloss, good whiteness, good discernibility, good liquidity, extraordinary hiding power and good coloring power, good weather resisting property and chalk resistance performance. It has the highest refractive index. Titanium dioxide melts at 1843 °C and its density is 4.23gm/cm3.
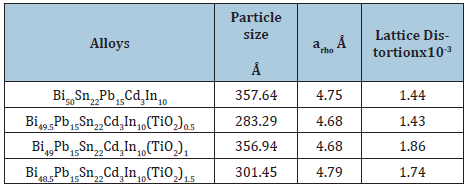
MicrostructureSn82-xBi15Zn3Ti2Ox (x= 0.3, 0.6, 0.9 and 1.2) alloys consist of tetragonal β-Sn phase, hexagonal Bi phase, Zn phase with undetected Ti2O or intermetallic compounds and solid solution from dissolved atoms that changed alloy matrix structure. Adding different ratio (0.5 to 1.5 wt. % at the expense of bismuth) from titanium oxide (TiO2) to Bi50-xPb15Sn22Cd3In10 alloy caused a change in matrix structure such as feature formed phases, crystal size, lattice distortion, lattice parameters, (a and c), and unit volume cell (V) [9] as listed in Table 1. Scanning electron micrographs, SEM, of Bi50- xPb15Sn22In10Cd3(TiO2)x (x=0 to 1.5 wt.%) alloys show heterogeneous structure (different feature for formed phases with different grain size beside a solid solution) as shown in Figure 1 which agreed with x-ray analysis.
Figure 1: SEM of Bi50-xPb15Sn22In10Cd3(TiO2)x alloys [9].

Table 1: Crystal size, lattice parameters and lattice distortion of rhombohedral Bi phase [9].
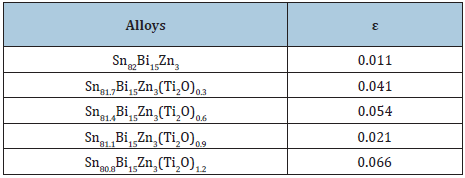
Sn82-xBi15Zn3Ti2Ox (x= 0.3, 0.6, 0.9 and 1.2) alloys consist of tetragonal β-Sn phase, hexagonal Bi phase, Zn phase with undetected Ti2O or intermetallic compounds and solid solution from dissolved atoms that changed alloy matrix structure. From SEM, Figure 2, adding Ti2O to Sn82Bi15Zn3 alloy caused a change in its matrix microstructure (quantity, size and orientation of formed phases) [24]. Lattice microstrain, ε, determined from draw the relation between full width half maximum, FWHM, and 4tanθ using Williamson and Hall equation. Lattice microstrain of Sn82Bi15Zn3 alloy increased after adding titanium dioxide as listed in Table 2.
Table 2: Lattice microstrain of Sn82-xBi15Zn3Ti2)x alloys [24].
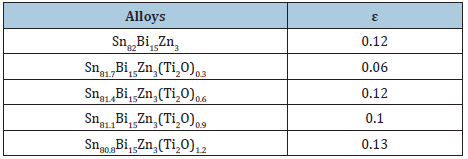
Sn60Bi30-xSb6Zn4Cu0.7Ti2Ox (x=0, 0.3, 0.6, 0.9 and 1.2) alloys consist of tetragonal β-Sn phase, hexagonal Bi phase, Zn phase, undetected phases such as Ti2O or Sb or Cu or intermetallic compounds and solid solution from dissolved atoms [26]. Lattice microstrain of Sn60Bi30Sb6Zn4Cu0.7 alloys varied after adding titanium oxide as listed in Table 3. SEM of Sn60Bi30-xSb6Zn4Cu0.7Ti2Ox alloys show heterogeneous structure from formed phases in matrix structure as shown in Figure 3 which agree with x-ray analysis.
Table 3: Lattice microstrain of Sn82-xBi15Zn3Ti2)x alloys [24].
The Sn76Al10Bi10-xCu2Zn2(TiO2)x (x=0.5 to 1.5 wt.%) alloys consist of tetragonal β-Sn phase and hexagonal Bi phase with formed a solid solution or undetected phases. The crystallinity, crystal size and orientations for formed phases of Sn76Al10Bi10Cu2Zn2 alloy changed after adding titanium oxide nanoparticles [26]. The estimated crystal size was given through measured diffraction pattern broadening by Scherer formula. Crystal size of β-Sn phase in Sn76Al10Bi10Cu2Zn2 alloy varied after adding titanium dioxide as presented in Table 4. Lattice microstrain of Sn76Al10Bi10Cu2Zn2 alloy decreased after adding titanium oxide up to 1wt. % and then increased as listed in Table 4. The Sn76Al10Bi9Cu2Zn2(TiO2)1 alloy has lower microstrain value. SEM show that adding different ratio from TiO2 nanoparticles to Sn76Al10Bi10Cu2Zn2 alloy changed the shape and size of dendrite structure and disturbed dissolved atoms as grains in it as seen in Figure 4. SEM analysis for used alloys shows heterogeneity structure and that is agreed with x-ray analysis [26].
Figure 2: SEM of Sn82-xBi15Zn3Ti2Ox alloys [24].

Figure 3: SEM of Sn60Bi30-xSb6Zn4Cu0.7Ti2Ox alloys [25].

Figure 4: SEM of Sn76Al10Bi10-xCu2Zn2(TiO2)x alloys [26].
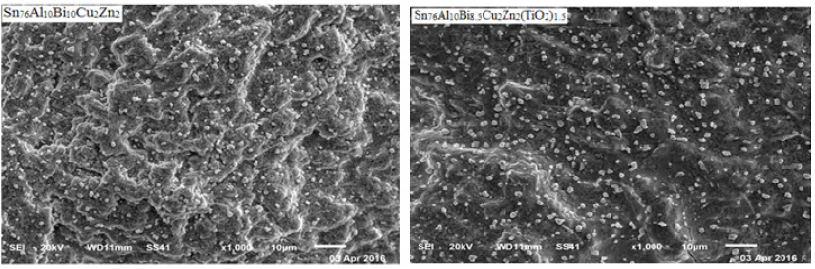
Table 4: Crystal size of β-Sn and lattice microstrain in Sn76Al10Bi10-xCu2Zn2(TiO2)x alloys [26].

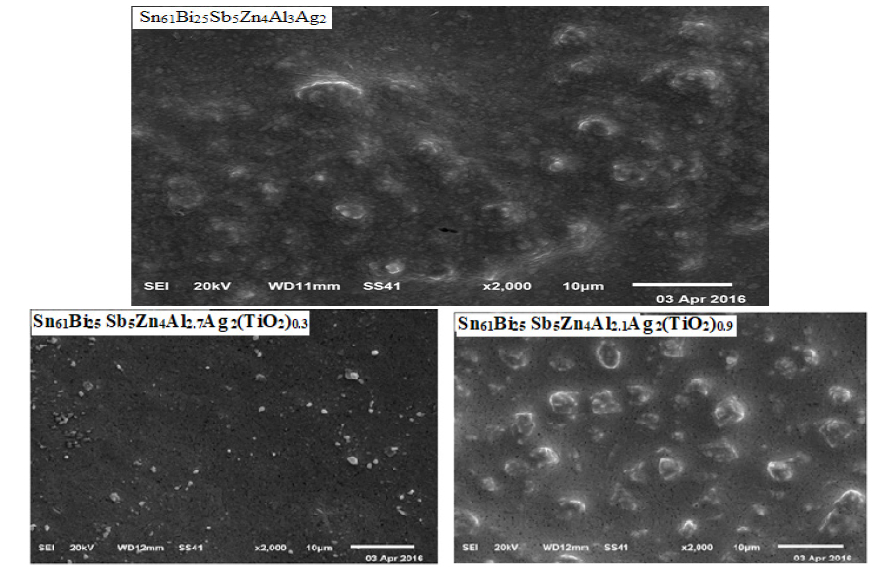
Sn61Bi25Sb5Zn4Al3-xAg2(TiO2)x (x=0.3 to 1.2 wt.%) alloys consist of β-Sn phase, hexagonal Bi phase and dissolved Sb, Zn, Al, Ag atoms with TiO2 nanoparticles or undetected phases in matrix [27]. Crystal size of β-Sn phase in Sn61Bi25Sb5Zn4Al3Ag2 alloy varied after adding TiO2 nanoparticles as listed in Table 5. Sn61Bi25 Sb5Zn4Al2.4Ag2(TiO2)0.6 alloy has higher lattice microstrain and lower β-Sn phase crystal size. SEM of Sn61Bi25Sb5Zn4Al3-xAg2(TiO2)x alloys [27], Figure 5, show that the matrix structure of Sn61Bi25Sb5Zn4Al3Ag2 alloy changed after adding TiO2 which agree with x-ray analysis and affected all measured properties.
Figure 5: SEM of Sn61Bi25 Sb5Zn4Al3-xAg2(TiO2)x alloys [27].
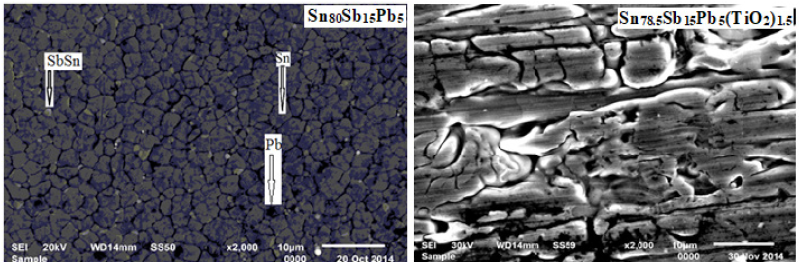
Sn80Sb15Pb5 alloy consists of β-Sn and SbSn intermetallic phases. After adding different ratio of titanium oxide (TiO2) nanoparticles to Sn80Sb15Pb5 alloy, SbSn intermetallic phase disappeared with changing matrix microstructure [29]. Lattice parameters, (a and c), unit volume cell (V) and crystal size (t) of β-Sn phase in Sn80-xSb15Pb5(TiO2)x (x= 0, 0.5, 1 and 1.5 wt. %) alloys are determined and then recorded in Table 6. Little variation occurred in lattice parameters and unit volume of β-Sn in Sn80-xSb15Pb5(TiO2)x alloys but a major variation in β-Sn crystal size after adding titanium oxide nanoparticles. Scanning electron micrographs, SEM, of Sn80Sb15Pb5 and Sn78.5Sb15Pb5(TiO2)1.5 alloys show heterogeneous structure as shown in Figure 6 and that agreed with x-ray analysis. Adding TiO2 caused a change in matrix microstructure of Sn80Sb15Pb5 alloy.
Table 5: Crystal size of β-Sn and lattice microstrain in Sn61Bi25sb5Zn4Al3-xAg2(TiO2)x alloys [27].
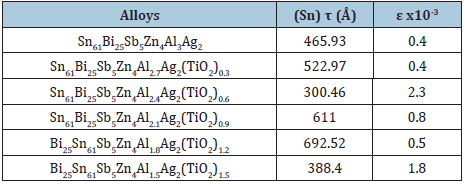
Table 6: Matrix parameters (a, b, c, V and t) of Sn80-xSb15Pb5(TiO2)x alloys [28].
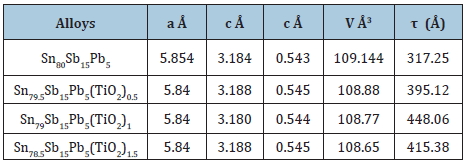
Figure 6: SEM of Sn80-xSb15Pb5(TiO2)2 alloys [28].
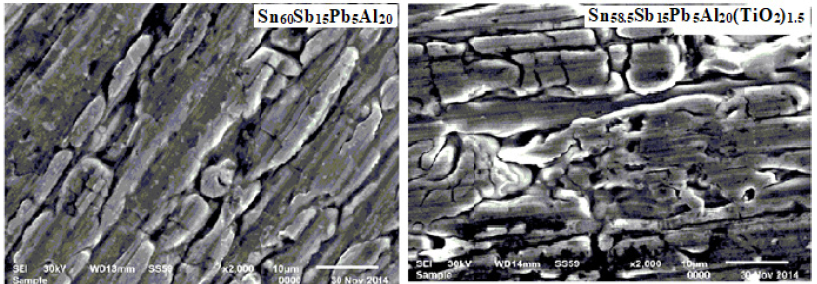
Sn60-xAl20Sb15Pb5(TiO2)x (x= 0.5, 1 and 1.5 wt.%) alloys consist of β-Sn, Pb and SbSn intermetallic phases [28]. The determined lattice parameters, unit volume cell and crystal size of β-Sn phase in Sn60-xAl20Sb15Pb5(TiO2)x recorded in Table 7. A little variation happened in lattice parameters and unit volume of β-Sn in Sn80-xSb15Pb5(TiO2)x alloys after adding titanium oxide nanoparticles but a major variation occurred in β-Sn crystal size. That is because TiO2 nanoparticles dissolved in matrix alloy formed a solid solution and resultant in disappeared or appeared phases with changed the shape of rest phases. Dissimilar structure for Sn60Sb15Pb5Al20 and Sn58.5Sb15Pb5Al20(TiO2)1.5 alloys are shown in scanning electron micrographs, Figure 7, which agreed with x-ray analysis. That is meant adding TiO2 caused a change in matrix microstructure [28].
Figure 7: Sn60-xAl20Sb15Pb5(TiO2)x alloys [28].
Sn645-xAg3.5Bi30In2(TiO2)x (x=0.5, 1 and 1.5 wt. %) alloys consist of β-Sn, Ag3Sn and hexagonal Bi phases [30]. That is meant that in atoms and TiO2 nanoparticles dissolved in alloy matrix forming a solid solution with changing microstructure. Lattice parameters, unit volume cell and crystal size of β-Sn in Sn645-xAg3.5Bi30In2(TiO2)x alloys are determined and then presented in Table 8. Unit cell volume of β-Sn in Sn645-xAg3.5Bi30In2(TiO2)x alloys decreased but lattice parameter, a, and crystal size varied after adding TiO2 nanoparticles. The internal lattice microstrain of Sn645-xAg3.5Bi30In2(TiO2)x alloys also was determined then show in Table 8. Lattice strain of Sn645-xAg3.5Bi30In2(TiO2)x increased with increasing TiO2 nanoparticles ratio.
Table 7: Matrix parameters (a, b, c, V and τ) of Sn60-xAl20Sb15Pb5(TiO2)x alloys [28]
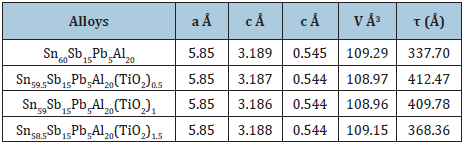
Table 8: Matrix parameters (a, c, V, τ and ε) of Sn645-xAg3.5Bi30In2(TiO2)x alloys [29].

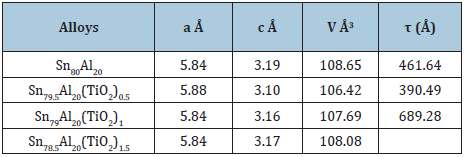
Sn87-xSb10Pb3(TiO2)x (x=0.5, 1 and 1.5 wt. %) alloys consist of β-Sn, Pb/or Sb and SbSn intermetallic phases [31]. Adding TiO2 to Sn87Sb10Pb3 alloy caused a change in Sn matrix structure such as lattice parameters and formed crystal structure (crystallinity, crystal size and the orientation) as seen in Table 9. That is because TiO2 nanoparticles dissolved in Sn matrix formed a solid solution and other accumulated particles formed a trace of phases. Also Sn80-xAl20(TiO2)x (x=0.5, 1and 1.5 wt.%) alloys consist of β- Sn and Al phases [31]. Adding TiO2 to Sn80Al20 alloy caused a change in Sn matrix such as lattice parameters and formed crystal structure (crystallinity and crystal size) as seen in Table 10.
Table 9: Matrix parameters (a, c, V and τ) of Sn645-xAg3.5Bi30In2(TiO2)x alloys [30].
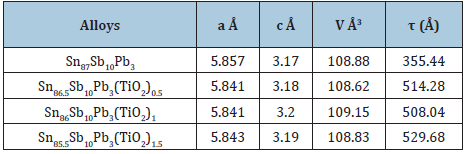
Table 10: Matrix parameters (a, c, V and τ) of Sn80-xSb15Pb5(TiO2)2 alloys [30].
Thermal properties
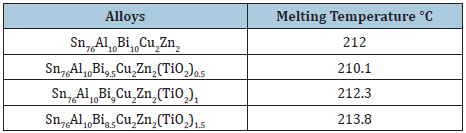
Measurement of thermal parameters is very inertial for their applications, particularly in the construction of devices. The melting temperature value of Bi50-xPb15Sn22In10Cd3(TiO2)x alloys increased after adding TiO2 nanoparticles which dependent on alloy compositions or alloy structure as presented in Table 11. The melting temperature value of Sn82Bi15Zn3 alloy is decreased after adding different ratio from titanium oxide as listed in Table 12. The melting temperature value of Sn60Bi29Sb6Zn4Cu0.7 alloy is varied after adding different ratio from titanium oxide as shown in Table 13. But no significant effect on melting temperature value of Sn76Al10Bi10Cu2Zn2 alloy after adding titanium dioxide nanoparticles as presented in Table 14.
Table 11: Melting temperature of Bi50-xPb15Sn22In10Cd3(TiO2)x alloys [9].
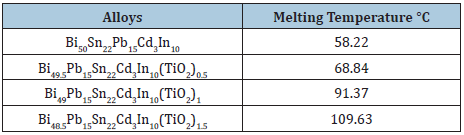
Table 12: Melting temperature of Sn82-xBi15Zn3Ti2Ox alloys [25].

Table 13: Melting temperature of Sn60Bi30-xSb6Zn4Cu0.7(Ti2O)x alloys [26].
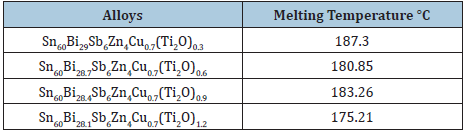
Table 14: Melting temperature of Sn76Al10Bi10-xCu2Zn2(TiO2)x alloys [27].
Melting temperature of Sn61Bi25Sb5Zn4Al3Ag2 alloy varied after adding TiO2 nanoparticles as listed in Table 15. It is increased up to 0.9 wt. % TiO2 nanoparticles and then it’s decreased. Melting temperature of Sn80Sb10Pb5 alloy decreased after adding TiO2 nanoparticles as presented in Table 16. Melting temperature of Sn60Al20Sb15Pb5 alloy varied after adding TiO2 nanoparticles as presented in Table 17. That is because TiO2 nanoparticles due a change in matrix microstructure of Sn60Al20Sb15Pb5 alloy. But melting temperature of Sn64.5-xAg3.5Bi30In2(TiO2)x (x=0.5, 1 and 1.5 wt. %) alloys varied after adding TiO2 nanoparticles as listed in Table 18.
Table 15: Melting temperature of Sn61Bi25Sb5Zn4Al3-xAg2(TiO2)x alloys [28].

Table 16: Melting temperature of Sn80-xSb15Pb5(TiO2)x alloys [29].
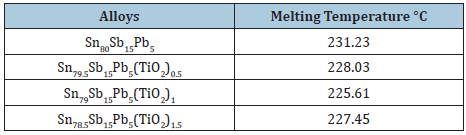
Table 17: Melting temperature of Sn60-xAl20Sb15Pb5(TiO2)x alloys [29].
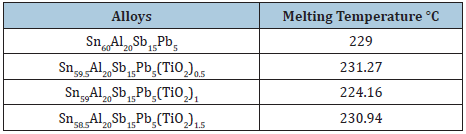
Table 18: Melting temperature of Sn645-xAg3.5Bi30In2(TiO2)x alloys [30].

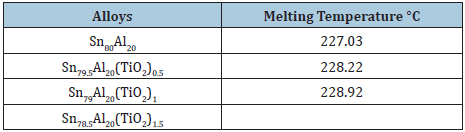
Melting temperature of Sn87Sb10Pb3 alloy decreased after adding TiO2 nanoparticles as shown in Table 19. The melting temperature of Sn80xAl20(TiO2)x (x= 0.5 to 1.5 wt.%) alloys are listed in Table 20. Little increased in melting temperature of Sn80Al20 alloy after adding TiO2 nanoparticles.
Table 19: Melting temperature of Sn645-xAg3.5Bi30In2(TiO2)x alloys [31].

Table 20: Melting temperature of Sn80-xAl20(TiO2)x alloys [31].
Mechanical properties
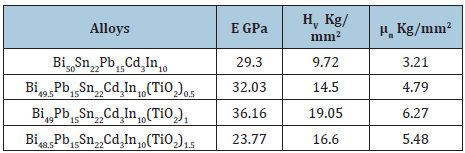
A significant change in elastic modulus of Bi50-xPb15Sn22Cd3In10(TiO2)x (x=0.5 to 1.5 wt.%) alloys with adding different ratio from TiO2 nanoparticles. Vickers hardness and calculated maximum shear stress of Bi50Pb15Sn22Cd3In10 alloy increased after adding (TiO2) as listed in Table 21.
Table 21: Elastic modulus, hardness and maximum shear stress of Bi50-xPb15Sn22In10Cd3(TiO2)x alloys [24].
Vickers hardness and calculated maximum shear stress of Sn76Al10Bi10-xCu2Zn2(TiO2)x alloys at 10 gram force and indentation time 5 sec are presented in Table 22. Vickers hardness value of Sn76Al10Bi10Cu2Zn2 alloy varied after adding titanium oxide nanoparticles.
Table 22: Vickers hardness and maximum shear stress of Sn76Al10Bi10-xCu2Zn2(TiO2)x alloys [27].
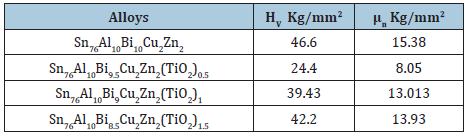
Table 23: Elastic modulus of Sn80-xSb15Pb5(TiO2)x alloys [29].
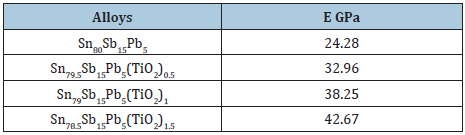
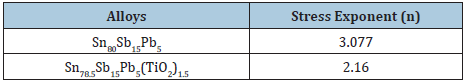
Elastic modulus value of Sn80-xSb15Pb5(TiO2)x (x= 0, 0.5, 1 and 1.5 wt. %) alloys is listed in Table 23. Elastic modulus of Sn80Sb15Pb5 alloy increased after adding TiO2 nanoparticles because it’s dissolved in alloy matrix. The stress exponent values of Sn80-xSb10Pb5(TiO2)x alloys which calculated using Mulheam-Tabor method are given in Table 24. These exponent values are in the range of 2.16 to 3.07 depending on the composition of alloy and that agreed with the pervious results [32,33]. The change in stress exponent values are attributable to microstructural features (such as change in lattice parameters, solid solution, size and distribution of strengthening phases, intermetallic phases) and that is agree with the pervious results [34].
Table 24: Stress exponent (n) of Sn80-xSb15Pb5(TiO2)x alloys [29].
Elastic modulus of Sn60-xAl20Sb15Pb5(TiO2)x (x= 0.5, 1 and 1.5 wt.%) alloys are registered in Table 25. Elastic modulus of Sn60Al20Sb15Pb5 alloy varied after adding TiO2 nanoparticles. The stress exponent Sn60Al20Sb15Pb5 alloy decreased after adding TiO2 nanoparticles as shown in Table 26. These exponent values are in the range of 2.44 to 5.75 depending on the composition of used alloy. Elastic modulus and Vickers hardness values of Sn64.5Ag3.5Bi30In2 alloy decreased after adding TiO2 nanoparticles as shown in Table 27.
Table 25: Elastic modulus of Sn60-xAl20Sb15Pb5(TiO2)x alloys [29].
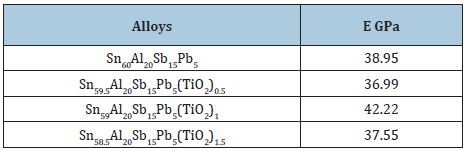
Table 26: Stress exponent (n)of Sn60-xAl20Sb15Pb5(TiO2)x alloys [29].
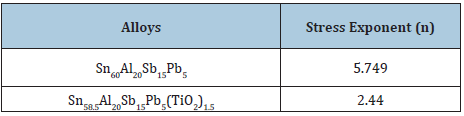
Table 27: Elastic modulus and Vickers hardness of Sn64.5-xAg3.5Bi30In2(TiO2)x alloys [30].
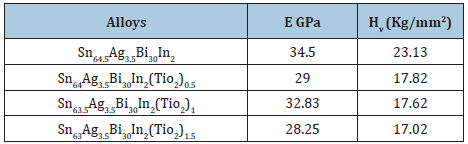
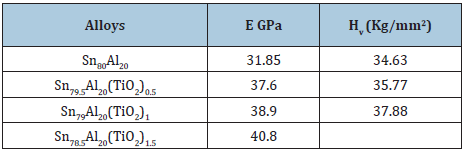
The elastic constants are directly related to atomic bonding and structure. Elastic modulus and Vickers hardness values of Sn87-xSb10Pb3(TiO2)x alloys are listed in Table 28. Elastic modulus and Vickers of Sn87Sb10Pb3 alloy increased after adding different ratio from TiO2 nanoparticles. Elastic modulus and Vickers hardness values of Sn80-xAl20(TiO2)x (x= 0.5 to 1.5 wt. %) alloys are listed in Table 29. Elastic modulus of Sn80Al20 alloy increased after adding different ratio from TiO2 nanoparticles. Also, little variation occurred in Vickers hardness at 10-gram force and indentation time 5 sec of Sn80Al20 alloy after adding TiO2 nanoparticles.
Table 28: Elastic modulus and Vickers hardness of Sn645-xAg3.5Bi30In2(TiO2)x alloys [31].

Table 29: Elastic modulus and Vickers hardness of Sn80-xAl20(TiO2)x alloys [31].
Corrosion parameters
The corrosion potential (ECorr), corrosion current (ICorr) and corrosion rate (CorrRate) of Sn82-xBi15Zn3Ti2Ox (x=0.3 to 1.2 wt. %) alloys are presented in Table 30. Corrosion rate and corrosion current of Sn82Bi15Zn3 alloy decreased after adding (Ti2O). The corrosion potential, corrosion current and corrosion rate of Sn60Bi30-xSb6Zn4Cu0.7(Ti2O)x (x= 0.3 to 1.2 wt.%) alloys are listed in Table 31. Corrosion rate and corrosion current of Sn60Bi30Sb6Zn4Cu0.7 alloy varied after adding different ratio from (Ti2O)x. Sn60Bi28.4Sb6Zn4Cu0.7(Ti2O)0.9 alloy has better corrosion resistance.
Table 30: Corrosion parameters of Sn82-xBi15Zn3Ti2Ox alloys [25].
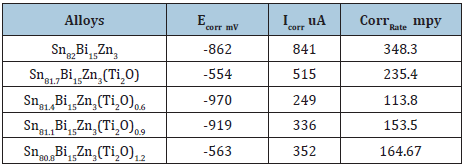
Table 31: Corrosion parameters of Sn60Bi30-xSb6Zn4Cu0.7(Ti2O)x alloys [26].
The corrosion current, corrosion potential and corrosion rate of Sn76Al10Bi10-xCu2Zn2(TiO2)x (x=0.5 to 1.5 wt.%) alloys in 0.25M HCl are presented in Table 32. Corrosion rate and corrosion current of Sn76Al10Bi10Cu2Zn2 alloy decreased after adding 0.5 and 1.5wt. % titanium oxide nanoparticles but increased after adding 1wt. %.
Table 32: Corrosion parameters of Sn76Al10Bi10-xCu2Zn2(TiO2)x alloys [27].
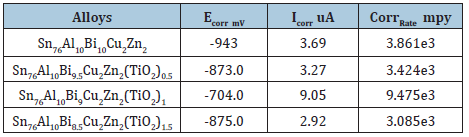
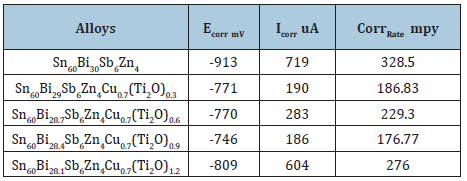
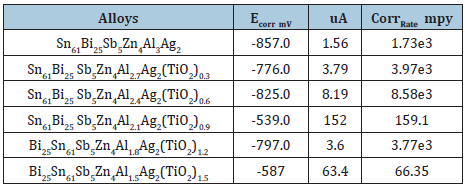
Table 33 presents the corrosion potential, corrosion current, and corrosion rate of Sn61Bi25Sb5Zn4Al3-xAg2(TiO2)x (x=0.3 to 1.2 wt.%) alloys. Corrosion rate of Sn61Bi25Sb5Zn4Al3Ag2 alloy varied decreased up to 0.9wt. % titanium dioxide nanoparticle and then increased with increasing the ratio of titanium dioxide nanoparticle. That is because adding titanium dioxide nanoparticle caused heterogeneous microstructure with affected on microsegregation and reactivity of atoms with HCl solution. The Sn61Bi25Sb5Zn4Al1.5Ag 2(TiO2)1.5 alloy has high corrosion resistance.
Table 33: Corrosion parameters of Sn61Bi25Sb5Zn4Al3-xAg2(TiO2)x alloys [28].
Conclusion
Adding titanium oxide which has low density, high refractive index with higher melting temperature to tin or bismuth-based alloys improved their properties due changed its matrix structure for make it used in different industrial applications.
References
- El-Bediwi AB, Samir R, Kamal M (2018) Electrochemical corrosion behavior, microstructure and soldering properties of tin based alloys. Material Science Research India 15(1): 12-22.
- El-Bediwi AB, Khalaf MD, Omar Kh M, Meaz TM (2017) Effect of modification structure on some properties and corrosion behavior of Bi-Sn-Pb-Cd alloys for shielding blocks. IJSRET 3(6): 820- 826.
- El-Bediwi AB, Bader S, Khalifa F (2016) Microstructure, electrochemical corrosion behavior and soldering properties of hexa and hepta bismuth-tin based alloy. Global Journal of Physics 4(3): 480- 495.
- El-Bediwi AB, El-Shishtawi NA, Abdullah MM (2016) Study microstructure, hardness, internal friction, electrochemical corrosion and thermal behavior of tin-aluminum-antimony or tin-aluminum-bismuth based alloys. Global Journal of Physics 4(3): 445- 460.
- El-Bediwi AB, Bader S, Khalifa F (2016) Effect of alloying elements on electrochemical corrosion behavior, microstructure, wettability and thermal performance of bismuth-tin based alloys. IJSRSET 2(2): 1267-1277.
- El-Bediwi AB, El-Shishtawi NA, Abdullah MM (2016) Electrochemical corrosion behavior, microstructure, mechanical and thermal performance of tin-aluminum based bearing alloys. IJSRET 2(2): 838-842.
- El-Bediwi AB, Jubair MM, Shalaby RM, Kamal M (2015) Effect of adding indium on wetting behavior, microstructure and physical properties of tin- zinc eutectic alloy. International Journal of Science and Engineering Applications 4(4): 186-190.
- El-Bediwi AB, El-Shafei A, Kamal M (2015) Study the effect of adding bismuth or bismuth-indium on structure and physical properties of eutectic tin-copper alloy. Global Journal of Physics 2(1): 67-78.
- El-Bediwi AB, Dawood F, Kamal M (2015) New hexa bismuth- tin based alloys for electrical fuse and nuclear applications. International Journal of Current Research 7(5): 16433-16439.
- El-Bediwi AB, Al-Bawee A, Kamal M (2015) Corrosion behavior and physical properties of modified tin-antimony bearing alloy. MSAIJ 13(4): 136-144.
- El-Bediwi AB, Dawood F, Kamal M (2015) Effect of quaternary addition on structure, electrical, mechanical and thermal properties of bismuth-tin-zinc rapidly solidified fusible alloy. Journal of Advances in Physics 7(3): 1952-1958.
- El-Bediwi AB, El-Shafei A, Kamal M (2014) Influence of adding bi\or bi-in on structure and required properties of tin-copper lead free solder alloy. International Journal of Engineering & Technology 14(6): 61-68.
- El-Bediwi AB, Kashita E (2013) Effects of alloying elements on physical properties of tin-antimony based lead free solder alloys. MSAIJ 9(5): 163-167.
- El-Bediwi AB (2005) Correlation of structure and properties of melt spun PbSn30Sb7Cu quaternary alloys. Cryst Res Technol 40(7): 688-691.
- El-Bediwi AB, El-Bahay MM (2004) Influence of sliver on structural, electrical, mechanical and soldering properties of tin-indium based alloys. Radiation Effect & Defects in Solids 159(2): 133-140.
- El-Bediwi AB (2003) Effects of micro -addition on structures and properties of rapidly solidified Sn-10%Sb alloy. Radiation Effect & Defects in Solids 158(7): 475-479.
- El-Bediwi AB (2002) Effect of ternary addition and cooling rate on the structure and properties of Lead-Tin based alloy. AMSE 75(3-4).
- Glazer J (1994) Microstructure and mechanical properties of Pb-free solder alloys for low-cost electronic assembly: A review. Journal of Electronic Materials 23(8): 693-700.
- Shiue RK, Tsay LW, Lin CL, Ou JL (2003) A study of Sn-Bi-Ag-(In) lead-free solders. J Mater Sci 38(6): 1269-1279.
- Osório WR, Peixoto LC, Garcia LR, Noël NM, Garcia A (2013) Microstructure and mechanical properties of Sn-Bi, Sn-Ag and Sn-Zn lead-free solder alloys. J Alloy Compd 572: 97-106.
- Shepelevich VG, Gusakova OV, Shcherbachenko LP (2013) Structure and properties of rapidly solidified Sn-58 wt % Bi foils. Inorganic Mater 49(7): 663-667.
- Esfandyarpour MJ, Mahmudi R (2011) Microstructure and tensile behavior of Sn-5Sb lead-free solder alloy containing Bi and Cu. Mate Sci Eng: A 530: 402-410.
- Mc Cormack M, Chen HS, Kammalott GW, Jin S (1997) Significantly improved mechanical properties of bi-sn solder alloys by ag- doping. J Electron Mater 26(8):954-958.
- El-Bediwi AB, Samir R (2018) Influence of titanium oxide on structure, corrosion and soldering properties of sn82bi15zn3 Res Dev Material Sci 6(1): 1-8.
- El-Bediwi AB, Samir R. Effect of titanium oxide nanopartiles on corrosion behavior and solder properties of penta tin- bismuth based
- El-Bediwi AB, El-Shishtawi NA, Abdullah MM (2016) Influence of titanium dioxide nanoparticles on microstructure, electrochemical corrosion behavior, mechanical and thermal properties of Sn-Al-Bi based alloy. International Journal of Applied Science and Biotechnology 4(3): 417-424.
- El-Bediwi AB, Bader S, Khalifa F (2016) Study the effect of titanium dioxide on microstructure, electrochemical corrosion parameters, thermal behavior and internal friction of hexa tin-bismuth based alloy. Mat Sci Ind J 14(13): 1-19.
- El-Bediwi AB, Grayb M, Kamal M (2015) Influence of titanium oxide on creep behavior, microstructure and physical properties of tin-antimony and tin-aluminum-antimony based bearing alloys. International Journal of Science and Engineering Applications 4(2): 64-70.
- El-Bediwi AB, El-Shafei A, Kamal M (2015) New tin-bismuth based lead free solder alloys with superior properties for industrial applications. International Journal of Current Research 7(6): 17305-17311.
- El- Bediwi AB, Abbas Al- Bawee, Kamal M (2015) Effect of titanium oxide on structure, bearing properties of tin-antimony-lead and tin-aluminum alloys. International Journal of Science and Engineering Applications 4(2): 46-53.
- Geranmayeh AR, Mahmudi R (2005) Power law indentation creep of Sn-5% Sb solder alloy. J Matter Sci 40(13): 3361-3366.
- Kangooie M, Mahmudi R, Geranmayeh AR (2010) Impression creep of a lead-free Sn-1.7Sb-1.5Ag solder reinforced by submicron-size al2o3 J Electr Matter 39(2): 215-222.
- Rani SD, Murthy GS (2004) Impression creep behaviour of tin based lead free solders. Mate Sci Techn 20(3): 403-408.
© 2019 Abu Bakr El- Bediwi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






