- Submissions

Full Text
Research & Development in Material Science
Material Aspects Pertaining to Hydrogen Production from Aluminum: Opinion
SP du Preez* and Bessarabov DG*
HySA, Faculty of Engineering, North-West University (NWU), South Africa
*Corresponding author: SP du Preez and Bessarabov DG, HySA Infrastructure, Faculty of Engineering, North-West University (NWU), Private Bag X6001, Potchefstroom 2520, South Africa
Submission: October 07, 2019;Published: October 15, 2019

ISSN: 2576-8840 Volume12 Issue1
Opinion
Background
The use of lightweight metals as hydrolyzing materials for hydrogen (H2) generation has attracted much attention in recent years. This is clearly evident when one considers the continuous stream of publications being made available in the public peer-reviewed domain on the subject. Metal-based hydrolyzing materials are combined with water, under certain conditions, to allow the hydrolysis reaction, yielding high-purity H2 on demand. Of the lightweight metals investigated, aluminum (Al) has shown significant potential as it is lightweight, relatively inexpensive, and abundant.
Reaction mechanisms
The complete hydrolysis of Al yields 1.36 L H2 per g Al under standard atmospheric conditions, according to the following reactions:
2Al+4H2O=2AlO (OH)+3H2
2Al+6H2O=2Al (OH)3+3H2
However, the hydrolysis of Al is prevented by a thin, coherent oxide layer on the surface of Al, which prevents contact between the underlying metallic Al and water. This oxide layer is generally removed via the following routes: (i) exposure to solution of extreme pH, ii) amalgamation with mercury/gallium, iii) thermal homogenization with certain metals (typically with metals with melting points below that of Al), and iv) mechanochemical processing with water soluble salts, metal oxides and/or certain metals (e.g., gallium, zinc, tin, indium, and/or bismuth). Of these methods, it is the mechanochemical processing of Al, which does not require the use of corrosive liquids and expensive/toxic elements, that appears most favorable. Advantages of its application include user/environmental friendliness and relatively low fabrication costs.
Mechanochemically processed Al
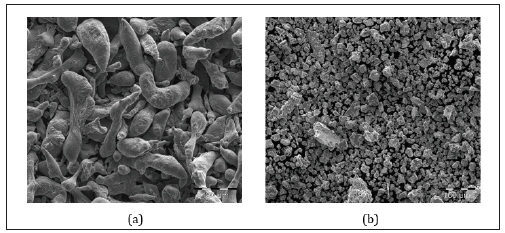
HySA Infrastructure (South Africa) has undertaken the fabrication of mechano-chemically processed Al composites using various activation compounds: bismuth, tin, and/or indium. These composites are highly reactive towards neutral pH water of various qualities (e.g., tap, filtered, deionized) [1-5]. Mechanochemical processing facilitates particle size reduction of the composite particles and the distribution of activation compounds throughout Al particles. When exposed to water, a large surface area and numerous microgalvanic cells between anodic Al and cathodic activation compounds affords these composites with high hydrolysis activity-yields of >99% H2 are achieved. Figure 1 illustrates the change in morphology of Al before and after processing.
The H2 generated from the hydrolysis of mechanochemically processed Al may be combined with proton exchange membrane fuel cells (PEMFCs) to convert the chemical energy of H2 into an electrical current. Because of the high purity of the generated hydrogen (more specifically, the absence of platinum-catalyst-poisoning carbon monoxide), its direct application in PEMFCs is acceptable without concern for catalyst degradation. The complete hydrolysis of 1kg of Al may generate the energy equivalent of approximately 4.4kWh worth of H2-approximately 50-60% of which may be converted to electrical energy (depending on the efficiency of the relevant PEMFC) [6,7]. A possible real-world application includes refueling of PEMFC-powered unmanned aerial vehicles (UAVs) in the field. A 16kg battery-powered UAV has an operational capacity of 5h, whereas a similar PEMFC powered system consuming gaseous or liquid H2 can operate for 24 to 48h [8]. A recent report by the US Army states the following: “The U.S. Army Research Laboratory plans to license its discovery of a nanogalvanic aluminum powder for H2 generation” [7]. The reasoning behind this is likely due to the low noise emissions of fuel cells, allowing stealthier operations. In addition to this, the relatively easy heat management of such a system can afford vehicles with low infrared signatures, when compared to traditional internal combustion engines used in military vehicles.
Figure 1:Field emission scanning electron microscopy images of Al particles: (a) before and (b) after mechanochemical processing using bismuth, tin, and/or indium as activation compounds.
Challenges
However, it is likely that suitable high-capacity reactor designs have yet to be realized as the hydrolysis of Al releases an appreciable amount of heat, which has a significant effect on the hydrolysis reaction kinetics. Al’s hydrolysis is significantly accelerated by in-situ generated reaction heat and it may cause operational complications. In addition to this, hydrolysis products (AlO(OH) and/or Al(OH)3) precipitate as a gel-like substances that may result is reactor complications, such as blockages.
Considering the energy required to convert Al hydroxides to alumina, and alumina to Al metal via the Hall-Heroult process, H2 generation from the hydrolysis of Al is a niche application. Nevertheless, this H2 generation route has significant potential for on-site and on-demand energy vector generation. It is recommended that further research into developing up-scaled processes for Al composite fabrication, fit-for-purpose reactors, and real-world applications should be carried out in the future.
Acknowledgment
The Department of Science and Innovation (DSI), South Africa, and HySA Infrastructure Centre of Competence at the North-West University (NWU), South Africa, are acknowledged for financial support through the KP5 program.
References
- Du Preez SP, Bessarabov DG (2017) Hydrogen generation by means of hydrolysis using activated Al-In-Bi-Sn composites for electrochemical energy applications. International Journal of Electrochemical Sciences 12: 8663-8682.
- Du Preez SP, Bessarabov DG (2017) Hydrogen generation of mechanochemically activated Al-Bi-In composites. International Journal of Hydrogen Energy 42(26): 16589-16602.
- Du Preez SP, Bessarabov DG (2018) Hydrogen generation by the hydrolysis of mechanochemically activated aluminum-tin-indium composites in pure water. International Journal of Hydrogen Energy 43(46): 21398-21413.
- Du Preez SP, Bessarabov DG (2019) The effects of bismuth and tin on the mechanochemical processing of aluminum-based composites for hydrogen generation purposes. International Journal of Hydrogen Energy 44(39): 21896-21912.
- Bessarabov DG, Du Preez SP (2018) Activation compounds for hydrogen generation, Netherlands Patent, Netherlands.
- Elitzur S, Rosenband V, Gany A (2015) Electric energy storage using aluminum and water for hydrogen production on-demand. International Journal of Applied Science and Technology 5(4): 112-121.
- (2018) Army plans to license nanogalvanic aluminum powder discovery.
- Osenar P, Sisco J, Reid C (2017) Advanced propulsion for small unmanned aerial vehicles: The role of fuel cell‐based energy systems for commercial UAVs. Pp. 1-15.
© 2019 SP du Preez. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






