- Submissions

Full Text
Research & Development in Material Science
Effective and Ideal Separation Factors at Sublimation Refining of Sm, Eu, Tm, Yb
Кravchenko AI*
National Science Center “Kharkov Institute of Physics and Technology”, Ukraine
*Corresponding author:Кravchenko AI, National Science Center “Kharkov Institute of Physics and Technology”, Kharkov, Ukraine
Submission: August 21, 2019;Published: September 10, 2019

ISSN: 2576-8840 Volume11 Issue4
Opinion
It is shown that the application of ideal separation factor in calculations of hightemperature sublimation refining of Sm, Eu, Tm and Yb has confined character: in considered base-impurity systems a difference between effective (β) and ideal (βi) separation factors increases with deviation of βi from unity and can be very significant; the measurable difference (in limited of a one order) between β and ideal βi is observed at deviation of βi from unity not more than 2 orders. The discrepancies between the effective and ideal separation factors in evaporative refining processes (distillation and sublimation at low impurity content) are not associated with the chemical interaction of the components.
Sublimation is one of the main methods for obtaining high-purity substances (as well as distillation and crystallization), in connection with which interest is shown in the theory of sublimation refining [1,2]. It was shown that high-temperature sublimation (at a temperature near the melting point) is described by the same simple equations as distillation.
One of the questions of the theory of distillation and sublimation is the question of the applicability of the ideal separation coefficient βi in the calculations of these processes. The interest in using βi in the calculations of distillation and sublimation refining of simple substances is related to the fact that βi values are known for most binary systems in a wide temperature range - since vapor pressures of almost all chemical elements are known [1], while values of the effective separation coefficient β for real distillation or sublimation processes depend on many factors and cannot be calculated before the experiment (βi=pi/p, where p and pi are the vapor pressure of the base and the impurity, respectively).
Using the basic equations of distillation, an analysis of the experimental data on the refining of a number of substances was carried out. A comparison was made between β and βi in the refining of the base-impurity systems, in which Cd, Ga, Mn, Pb, S, Sb, Se, Te, Zn served as the basis for distillation (about 40 systems) and As, Cr, Mg, Mn for high-temperature sublimation (about 20 systems) [2]. It was shown that the applicability of βi in the calculations of the distillation and sublimation refining of these systems is limited: the divergence of β and βi increases with deviation of βi from unity and can be quite significant; a moderate divergence of β and βi (within the same order of magnitude) is observed when the values of βi deviate from unity by no more than 3 orders of magnitude (in separate systems β/βi~1 and βi~1).
In pursuit of the fullness of knowledge about evaporative refining processes, attention was drawn to experimental data on the sublimation of Sm, Eu, Tm and Yb [3] - data that were not considered in a previous study [2]. The evaporation of these substances (with an initial impurity content of ~10-5-10-2wt%) was carried out at temperatures close to their melting points (melting points Sm, Eu, Tm, and Yb are 1350, 1099, 1818, and 1097K, respectively).
The purpose of this work was to study the ratio of the effective and ideal separation factors for the sublimation of lanthanides Sm, Eu, Tm and Yb and to determine the applicability of the ideal separation factor in the calculations of the sublimation refining of these substances. According to the experimental data on the content of impurities in Sm, Eu, Tm and Yb during sublimation, the order of values of the effective separation coefficient β in various base-impurity systems was estimated. As in [2], only volatile impurities were considered. The calculations took into account that for real distillation and hightemperature sublimation processes (with a degree of distillation usually not more than 90%) in the approximate calculations β~C/ C0, where C is the impurity concentration in the condensate, C0 is the initial impurity concentration in the substance being refined [2]. To calculate βi, we used data on the vapor pressure of pure components at the indicated temperatures [4]. The results of calculations of β and βi are given in Table 1 (total - for 33 base-impurity systems).
Table 1:Separation factors at sublimation refining of Sm, Eu, Tm, Yb.
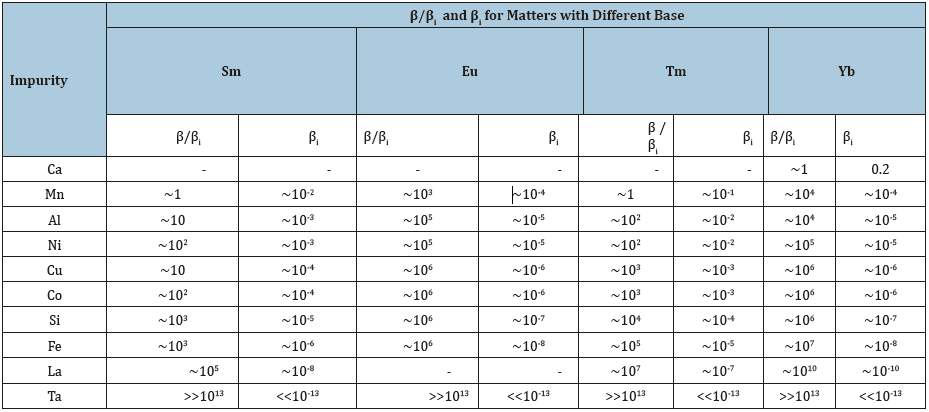
As in [2], it is noteworthy that the content of impurities does not decrease by more than 3 orders of magnitude - even when βi<<1 - and there is a relationship between β and βi. In general, the data obtained supplement the data of work [2], and the conclusion that can be made about the ratio of β and βi during sublimation of the considered systems does not contradict the conclusion of work [2] for distillation and sublimation.
It is important to note that, both during distillation and hightemperature sublimation (Table 1; [2]), β/βi ratio depends on βi, but does not depend on the nature of the components of the baseimpurity system: the same β/βi values are achieved in systems whose components are elements with different valences and are able to form chemical compounds with vapor pressure, significantly different from the vapor pressure of these elements. Therefore, the discrepancies between the effective and ideal separation coefficients in the evaporation refining processes (distillation and sublimation at low impurity content) are not due to chemical interaction of the components, but for other reasons (perhaps the entrapment of impurities by the main component vapor).
Conclusion
The applicability of the ideal separation ratio in the calculations of the high-temperature sublimation refining Sm, Eu, Tm and Yb is limited: in the considered base-impurity systems (with Ca, Mn, Al, Ni, Cu, Co, Si, Fe, La, Ta impurities) the divergence between the effective (β) and ideal (βi) separation factors increases with deviation of βi from unity and can be quite significant (by several orders of magnitude); a moderate divergence of β and βi (within the same order of magnitude) is observed when the values of βi deviate from unity by no more than 2 orders of magnitude.
The discrepancy between the effective and ideal separation coefficients in the evaporation refining processes (distillation and sublimation at low impurity content) is not due to chemical interaction of the components, but for other reasons (perhaps the entrapment of impurities by the main component vapor).
References
- Zhukov AI, Kravchenko AI (2017) Calculation of sublimation with allowance for impurity diffusion. Inorganic Materials 53(6): 648-653.
- Kravchenko AI (2016) Relationship between effective and ideal separation factors for distillation and sublimation. Inorganic Materials 52(4): 378-385.
- Ionov AM, Nikiforova TV, Rytus NN (1996) Aspects of the purification of volatile rare earth metals by UHV sublimation: Sm, Eu, Tm, Yb. Vacuum 47(6-8): 879-793.
- Nesmeyanov AN (1961) Davlenie para khimicheskikh elementov (Vapor Pressure of Chemical Elements), Akad Nauk SSSR, Moscow, Russia.
© 2019 Кravchenko AI. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






