- Submissions

Full Text
Research & Development in Material Science
Nanostructured Energy Characteristics of Hydrocarbon Hydrogen Containing Fuels
Korablev GA1*, Strelkov SM1, Khokhriakov NV1 and Zaikov GE2
1Izhevsk State Agricultural Academy, Russia
2NM Emanuel Institute of Biochemical Physics, Moscow
*Corresponding author:Korablev GA, Izhevsk State Agricultural Academy, Russia
Submission: May 23, 2019;Published: June 03, 2019

ISSN: 2576-8840 Volume11 Issue1
Abstract
The consistent calculations of bond energy in cluster water nanostructures have been performed following the P-parameter methodology and quantum-mechanical methods. The formation of high energy bonds in the process of hydrocarbon hydrogen containing fuel preparation has been explained.
Keywords: Spatial-energy parameter; Cluster water nanostructures; High energy bonds
Introduction
Water plays the ambiguous role in hydrocarbon fuel of internal combustion engines. On the one hand, simple dilution of petroleum or diesel fuel with water can significantly deteriorate technological characteristics of the fuel. As soon as water drops get into cylinders, the following happens: in the compression stroke when both valves are closed, the piston bears against the water plug when moving upwards. The pressure inside the cylinder increases multiply. The engine tries to bring the connecting rod to the upper position, continuing the cycle. In fact, the pistons in one or several cylinders stop at once, and the crankshaft which continues rotating takes enormous loads. It bends connecting rods, breaks piston pins and often breaks down itself. On the other hand, optimal water content in hydrocarbon fuel is defined by the standard technological norm of such fuel mixture which is prepared by a special technique. Moreover, based on the invention patent [1] water containing fuel can have the potential energy of 1/3 from energy unit of ВТИ - petroleum, and nevertheless the engines produce the same power as with additional amount of petroleum by the mass equaled to the mass of water added. And you not only have the power gain, but you also benefit in fuel technological characteristics, such as fire safety, octane number, application temperature limits, possibility to use cheaper fuels, etc. Such specificity of technological processes is ultimately defined by the mechanism of physic-chemical transformations occurring on atom-molecular level. In this investigation their possible evaluations are studied based on the concept of spatial-energy parameter (Р-parameter).
Formation of high energy bonds in fuel mixture
Practical use of hydrogen containing fuel is possible only if a number of conditions are fulfilled:
1. Introduction of complex additives into the fuel, with alcohols and so-called “hydrogen catalyst” content having the primary meaning.
2. Such additives are mixed following the special technique - first, by separate fractions, and in the end all the mixture is intensively stirred by a hydraulic cutting pump (hydraulic shears).
According to the author [1], “hydrogen catalyst” contributes to active dissociation of water molecules with the formation of hydrogen and oxygen which burn in the engine chamber afterwards. But is not clear how during such a short combustion time of the given amount of mixture introduced into the chamber, water dissociation in this volume and burning of its products can take place. Moreover, as a result of water dissociation by the reaction Н2О=Н++ОН– the direct oxygen release is not observed. Obviously, other important mechanisms of physic- chemical transformation of energy are involved. For instance, it is known that as a result of biochemical reactions in the presence of certain ferments the synthesis of ATP molecule can take place whose potential energy increases due to the formation of special high energy bonds.
It is possible that similar processes take place during the formation of burning mixture of this type of fuel when nanoclusters in the form of fullerenes can be formed under certain technological conditions. First of all, this is aided by the introduction of alcohols into the fuel mixture that results in the formation of fullerene, for example, С60(ОН)10. Therefore, the addition of alcohols (up to 20%) just corresponds to the ratio of molar masses of hydroxyl groups ОН– and carbon atoms. At the second stage of fuel preparation, high energy bonds are formed in the systems С60(ОН–)-n(Н2О), first of all, due to the introduction of “hydrogen catalyst” into the mixture, and besides, when filtering water through the coal filter which contributes to the extraction of nanostructured formations of carbon atoms into the mixture.
Similar to АТP hydrolysis which is accompanied by the release of chemical bond energy, the breakage of high energy bonds and heat energy release occur in hydrogen containing fuel when it is burning in the engine chamber. The physic-chemical mechanism of the formation of energy saturated bonds in this system is given below.
Investigation technique
The value of the relative difference of P-parameters of interacting atom-components-coefficient of structural interaction α was used as the main numerical characteristic of structural interactions in condensed media [2]:
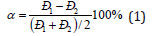
Applying the reliable experimental data, we obtain the nomogram of the dependence degree of structural interactions upon coefficient α -unified for the wide range of structures (no Figure is available). This approach allows evaluating the degree and directedness of structural interactions of phase formation, isomorphism and solubility processes in multiple systems, including molecular ones. In particular, the features of cluster formation in the system СаSO4 -H2O have been investigated [3].
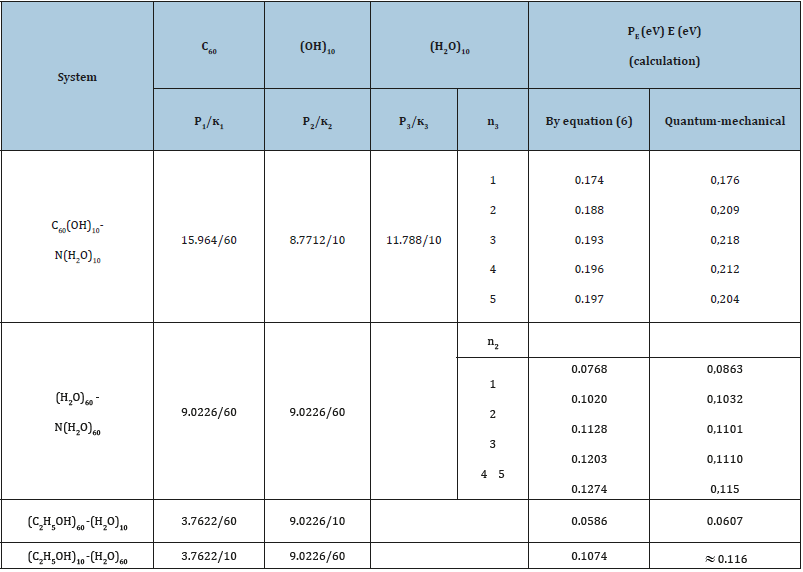
To evaluate the directedness and degree of phase formation processes [1] the following equations are used:
1. Initial values of P-parameters:
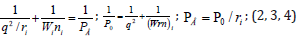
here: Wi -electron orbital energy [4]; ri-orbital radius of i orbital [5]; q = Z ∗ /n ∗ -by [6,7]; ni-number of electrons of the given orbital, Z ∗ and n ∗ -effective nucleus charge and effective main quantum number. Р0 is called as spatial-energy parameter, and РE-effective Р-parameter.
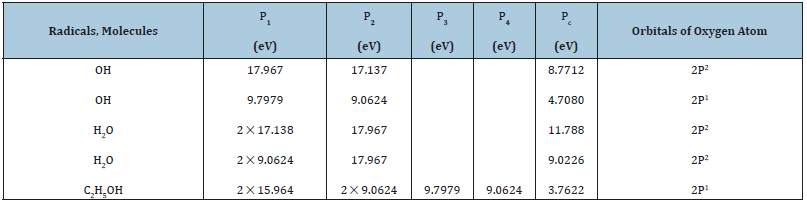
Table 1:Р-parameters of atoms calculated via the electron bond energy.
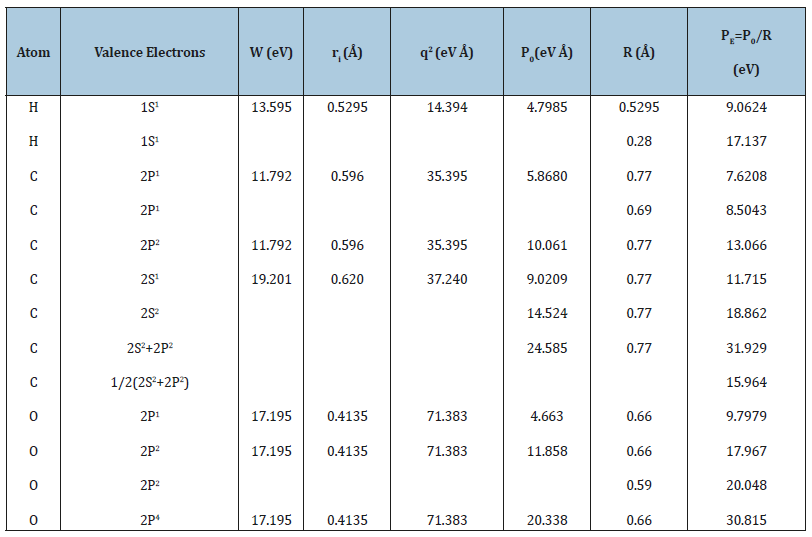
The calculation results by equations [2-4] for a number of elements are given in Table 1, from which it is seen that for hydrogen atom the values of РE-parameters substantially differ at the distance of orbital (ri) and covalent (R) radii. The hybridization of valence orbitals of carbon atom is evaluated as the averaged value of P-parameters of 2S2 and 2P2- orbitals.
2. Values of Рс-parameter in binary and complex structures:
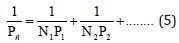
where N-number of homogeneous atoms in each subsystem.
The results of such calculations for some systems are given in Table 2.
Table 2:Structural Рс-parameters.
3. Bond energy (Е) in binary and more complex systems
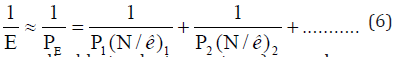
Here (as applicable to cluster systems) к1 and к2 -number of subsystems forming the cluster system; N1 and N2-number of homogeneous clusters [7]. So, for С060(OH)10 к1 = 60, к2 =10.
Calculations and comparisons
It was assumed that structural-stable water cluster (H2O) can have the same static number of subsystems (к) as the number of subsystems in the system interacting with it [8]. For example, the water cluster n (H2O)10 is interacting with fullerene [С6OH]10.
Similarly, with cluster [C6OH)10 the formation of cluster [(C2H5OH)6-H2O]10 is apparently possible, which corresponds to the system (C2H5OH)60-(H2O)10. The interaction of water clusters was considered as the interaction of subsystems (H2O)60-N(H2O)60 [9].
Based on such concepts, the bond energies in these systems are calculated by equation (6), the results are given in Table 3. To compare, the calculation data obtained by Khokhriakov NV with quantum-chemical techniques [10] are given. Both techniques produce consistent values of bond energy (in eV). Besides, the methodology of Р-parameter allows explaining why the energy of cluster bonds of water molecules with fullerene С060(OH)10 2 times exceed the bond energy between the molecules of cluster water (Table 3). In accordance with the nomogram, the phase formation of structures can take place only if the relative difference of their P-parameters (α ) is under 25-30%, and the most stable structures are formed when α <6-7%.
Table 3:Calculation of bond energy - Е (eV).
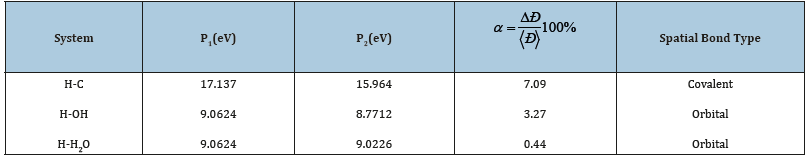
In Table 4 different values of coefficient α in systems H-C, H-OH and H-H2O are given, which are within 0.44-7.09(%). But in the system H-C for carbon and hydrogen atoms the interactions at the distances of covalent radii have been taken into account, and for other systems-at the distance of orbital radius. The interaction in system Н-С at the distances of covalent radius plays a role of fermentative action, which results in the transition of dimensional characteristics in water molecules from the orbital radius to the covalent one and formation of system С60(ОН)10-N(H20)10 with bond energy between the main components 2 times greater than between the water molecules (high energy bonds).
Table 4:Spatial-energy interactions in the system H-R, where R= C, (OH), H2O.
Thus, broad capabilities of water clusters to change their spatial-energy characteristics apparently explain all the diversity of structural properties of water in its different modifications, including the formation of high energy bonds in water containing fuel for internal combustion engines.
Conclusion
1. Results of bond energy calculations in water cluster nanostructures following the P-parameter methodology agree with quantum-mechanical methods.
2. Changes which can take place in spatial-energy characteristics of water clusters explain the formation of high energy bonds in the process of hydrocarbon fuel preparation.
3. Breaking of these bonds with the release of additional amount of heat energy occurs in the combustion chamber.
References
- Rudolph V. Gupperman. Patent No 5156114; US 1995.03.29.
- Korablev GA (2005) Spatial-energy principles of complex structures formation. Brill Academic Publishers and VSP, Leiden, Netherlands, pp. 1-426.
- Korablev GA, Yakovlev GI, Kodolov VI (2002) Some features of cluster formation in the system СаSO4- H2O. Chemical physics and mesoscopy 4(2): 188-196.
- Fischer CF (1972) Average-energy of configuration hartree-fock results for the atoms helium to radon. Atomic Data 4: 301-399.
- Waber JT, Cromer DT (1965) Orbital radii of atoms and ions. J Chem Phys 42(12): 4116-4123.
- Clementi E, Raimondi DL (1963) Atomic screening constants from SCF functions. J Chem Phys 38(11): 2686-2689.
- Clementi E, Raimondi DL (1967) Atomic screening constants from SCF Functions. J Chem Phys 47(4): 1300-1307.
- Korablev GA, Zaikov GE (2006) Energy of chemical bond and spatialenergy principles of hybridization of atom orbitals. J Applied Polymer Science 101(3): 2101-2107.
- Hodges MP, Wales DJ (2000) Global minima of protonated water clusters. Chemical Physics Letters 324(4): 279-288.
- Khokhriakov NV, Melchor F (2002) Electronic properties of contacts of ideal carbon nanotubes. Chemical physics and Mesoscopy 4(2): 261- 263.
© 2019 Korablev GA . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






