- Submissions

Full Text
Research & Development in Material Science
The Mechanism of Self-Reactivation of Cao-Based CO2 Adsorbents
Sufang Wu*, Fanfeng Pan, Hao Liu and Peiqiang Lan
College of Chemical and Biological Engineering, China
*Corresponding author:Sufang Wu, College of Chemical and Biological Engineering, China
Submission: May 06, 2019; Published: May 13, 2019

ISSN: 2576-8840 Volume10 Issue5
Abstract
The self-reactivation phenomenon of CaO-based CO2 adsorbents produced by high-temperature thermal pretreatment improves the durability of adsorbents in calcium looping process. The selfreactivation mechanisms of limestone and Nano-CaO-based adsorbents were explained as the conversion of hard skeleton to soft skeleton and the formation of cracks between Nano-grains respectively. The summarization of the recent researches about the triggering conditions is to provide reference for grasping and utilizing the self-reactivation of adsorbents.
Keywords:CO2 adsorption; Calcium looping; CaO-based adsorbent; Self-reactivation
Mini Review
Calcium looping (CaL) includes carbonation and regeneration process (Equation 1) has a wide use in flue gas and chemical process for CO2 capture [1-4]. Improving the cyclic stability of CaO-based adsorbents is a research hotspot in this field. Many researches focused on optimizing the pore structure [5-8], doping additives [9-11] and improving the skeleton stability of CaO-based adsorbents to reduce sintering [12].
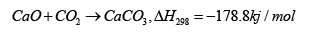
Manovic et al. [11]; [13,14] found that limestone exhibited an abnormal ‘self-reactivation phenomenon’ after thermal pretreatment at 900-1200 ℃ for 6-48h, in which the sorption capacity of the adsorbent rose with the increasing of initial cycle number (Figure 1). They proposed a ‘pore skeleton’ model to explain the mechanism of self-reactivation of limestone (Figure 2); [13]. The external skeleton in adsorbents which could react with CO2 was defined as ‘soft skeleton’ and the internal skeleton without carbonation was defined as ‘hard skeleton’. The pre-calcination enhanced sintering of the nascent CaO soft skeleton and the formation of a hard skeleton. During the subsequent CaL cycles, part of CaO in the hard skeleton could be converted into soft skeleton due to the deep carbonation reaction, which caused the increase in the number of active sites. It can be concluded that the self-reactivation mechanism of limestone is the conversion of hard skeleton to soft skeleton in adsorbents.
Figure 1:The self-reactivation phenomenon of limestone [11].
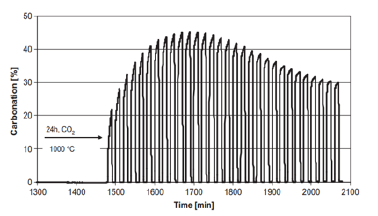
Figure 2:Pore-skeleton model of the self-reactivation mechanism of limestone [13].
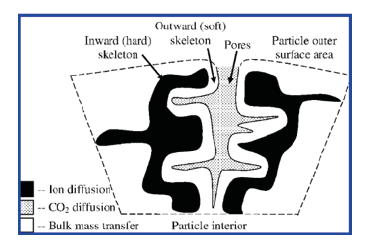
Figure 3:Grain-core-pore model of the self-reactivation mechanism of nano-CaO-based adsorbents [15].
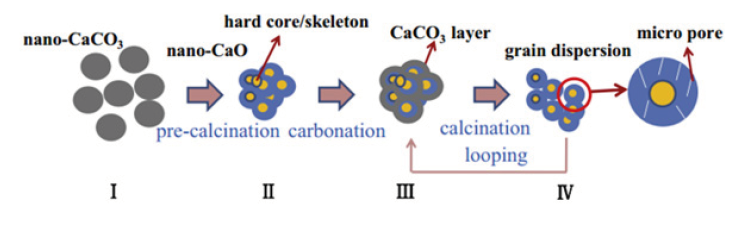
The self-reactivation mechanism of Nano-CaO-based adsorbents is different from that of limestone due to the different size of calcium precursors. S.F. Wu et al. [15] proposed a ‘grain-corepore’ model to explain the self-reactivation mechanism of Nano- CaO-based adsorbents (Figure 3). After nano-CaCO3 (I) was precalcined at high temperature for a prolonged period, the Nano-CaO particles were sintered and accumulated into larger particles with a hard core (II). During the first carbonation, larger CaO particles (II) were carbonated with CO2 and the reaction formed a thick CaCO3 layer (III). At the subsequent calcination stage, the decomposition of CaCO3 layer leaded to the release of CO2 and the return of part of Nano-CaO grains (IV), thereby increasing the number of active sites. In summary, the self-reactivation mechanism of Nano-CaO-based adsorbents is the formation of cracks between Nano-grains.
Based on these mechanism models, there are many researches focusing on the triggering conditions for self-reactivation of adsorbents. First, the self-reactivation triggering of the limestone adsorbent required severer thermal pretreatment operation conditions (high temperature, long time and CO2 atmosphere) to bring more hard skeletons [11], while the Nano-CaO-based sorbent was not resistant to sintering, requiring milder thermal pretreatment conditions [16]. Moreover, the increase of carbonation temperature and the prolongation of carbonation time as well as the reduction in the regeneration temperature were beneficial to the self-reactivation triggering of adsorbents [15]. Finally, the antisintering dopant was favorable for the self-reactivation triggering of adsorbents [13,15].
The self-reactivation phenomenon of CaO-based adsorbents induced by thermal pretreatment can effectively improve the sorption stability of adsorbents in CaL process, which has a practical application value. However, there are still some shortcomings in the recent studies of self-reactivation of CaO-based adsorbents. First, the reason for different effects brought by different kinds of dopants is not clear. Besides, in addition to focusing on the self-reactivation triggering conditions, there are few researches paying close attention to what factors affect the self-reactivation performance, which includes the degree of increase in sorption capacity during self-reactivation and the cyclic stability after self-reactivation.
References
- Rogelj J, Meinshausen M, Knutti R (2012) Global warming under old and new scenarios using IPCC climate sensitivity range estimates. Nat Clim Change 2(4): 248-253.
- Shimizu T, Hirama T, Hosoda H, Kitano K, Inagaki M, et al. (1999) A twin fluid-bed reactor for removal of CO2 from combustion processes. Chen Eng Res Des 77(1): 62-68.
- Ridha FN, Lu DY, Symonds RT, Champagne S (2016) Attrition of CaObased pellets in a 0.1MWth dual fluidized bed pilot plant for postcombustion CO2 capture. Powder Technol 291: 60-65.
- Wu SF, Li QH, Kim JN, Yi KB (2008) Porperties of a nano CaO/Al2O3 CO2 sorbent. Ind Eng Chem Res 47: 180-184.
- Alvarez D, Abanades JC (2005) Pore size and shape effects on the recarbonation performance of calcium oxide submitted to repeated calcination/recarbonation cycles. Energ Fuel 19: 270-278.
- Jiang L, Hu S (2016) Performance and carbonation kinetics of modified CaO-based sorbents derived from different precursors in multiple CO2 capture cycles. Energ Fuel 30(11): 9563-9571.
- Guo HX, Yan SL, Zhao YJ, Ma XB, Wang SP (2019) Influence of water vapor on cyclic CO2 capture performance in both carbonation and decarbonation stages for Ca-Al mixed oxide. Chem Eng J 359(1): 542- 551.
- Hu Y, Liu W, Sun J (2016) Structurally improved CaO-based sorbent by organic acids for high temperature CO2 capture. Fuel 167: 17-24.
- Azimi B, Tahmasebpoor M, Jimenez PE, Perejon A, Valverde JM (2019) Multicycle CO2 capture activity and fluidizability of Al-based synthesized CaO sorbents. Chem Eng J 358: 679-690.
- Ping HL, Wang Y, Wu SF (2016) Preparation of MgO-coated nano CaO using adsorption phase reaction technique for CO2 sorption. RSC Advances 6(47): 41239-41246.
- Manovic V, Anthony EJ, Loncarevic D (2009) CO2 looping cycles with CaO-based sorbent pretreated in CO2 at high temperature. Chem Eng Sci 64(14): 3236-3245.
- Bazaikin YV, Malkovich EG, Derevschikov VS, Lysikov AI, Okunev AG (2016) Evolution of sorptive and textural properties of CaO-based sorbents during repetitive sorption/regeneration cycles. Chem Eng Sci 152: 709-716.
- Manovic V, Anthony EJ (2008) Thermal activation of CaO-based sorbent and self-reactivation during CO2 capture looping cycles. environ. Sci Technol 42(11): 4170-4174.
- Manovic V, Anthony EJ (2009) CaO-based pellets supported by calcium aluminate cements for high-temperature CO2 capture. Environ Sci Technol 43(18): 7117-7122.
- Lan PQ, Wu SF (2015) Mechanism for self-reactivation of nano-CaObased CO2 sorbent in calcium looping. Fuel 143: 9-15.
- Wu SF, Zhu YQ (2010) Behavior of CaTiO3/Nano-CaO as a CO2 reactive adsorbent. Ind Eng Chem Res 49(6): 2701-2706.
© 2019 Sufang Wu . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






