- Submissions

Full Text
Research & Development in Material Science
Phosphorus-derived compounds for Li and Na ion batteries
Ramesh Kumar P1, Anteneh Marelign B2, Do Kyung Kim2 and Amin R1*
1 Qatar Environment and Energy Research Institute (QEERI), Qatar
2 Department of Materials Science and Engineering, Republic of Korea
*Corresponding author:Ruhul Amin, Qatar Environment and Energy Research Institute (QEERI), Qatar
Submission: April 04, 2019; Published: April 09, 2019

ISSN: 2576-8840 Volume10 Issue4
Abstract
In this review, progress in Phosphorus-based electrode materials for lithium, and sodium batteries, including Olivine, Tavorite, NASICON, Phosphite and phosphorene types, is briefly summarized. Such Phosphorus-based electrode materials will be attractive for next generation energy storage devices owning to its structural features which provide ultimate safety guarantee compared to other electrode materials.
Introduction
Rechargeable batteries have been paid remarkable attention and are regarded as the most promising energy storage system, especially for mobility (electric vehicle) and large-scale (gird level)) energy storage applications, owning to the high energy density, power density and cycle life compared to other storage systems. Noted that during the last 10 years, Lithium and sodium ion battery research has been focused on which is made possible to deliver low cost and high energy density devices. Considerable research efforts have been made to investigate on high voltage, high-capacity and stable electrode materials for alkali metal ion batteries. Overall electrochemical performance of battery is truly depends on the characteristic features of electrode materials. Currently, Phosphorus-based compounds such as, metallo-phosphates, pyrophosphates, fluorophosphates, Oxy-fluorophosphates, mixed phosphates and phosphites have been considered as thermally and chemically stable materials for alkali metal ion batteries [1,2]. These compounds have an open phosphate structure, which provides an easy motion of alkali metal ions and the strong bonding of oxygen atoms with phosphorus helps to avoid oxidation with the electrolyte. Furthermore, elemental phosphorus (P) was found to be electrochemically active and used as an alkali metal ion battery anode. The three-electrontransfer reaction of phosphorus with alkali metal provides an extremely high theoretical capacity of 2595mAhg−1, which is almost seven times higher than that of graphite. Therefore, these compounds have the ability to prevent an internal short circuit in the energy storage devices and high storage capacity; however, they have been suffering for low electronic & ionic conductivities and large volume expansion which are needed to be addressed for sustainable use of the materials.
Here we present a comprehensive mini review on current research progress that has been focused on the development of phosphates and phosphorus based materials for non-aqueous alkali metal ion battery electrodes. An overview of synthesis and electrochemical properties of polyanionic phosphates and phosphites versus Li & Na is provided.
Discussion
In 1997, Padhi et al. [3] demonstrate the electrochemical activity of LiFePO4 olivine structure based on the two-phase reaction LiFePO4 → FePO4 + Li+ + e−with a first-order phase transition and Fe3+/Fe2+ redox potential of 3.4V [3]. All Olivine LiMPO4 (M= Mn, Fe, Co and Ni) materials have low intrinsic electronic conductivity which are obstacles for the lithium ion motion in system. These drawbacks may be due to the (i) Li/M anti-site defects (ii) M ion on an Li site and a lithium vacancy and (iii) M3+ hole center (small polaron) and a lithium vacancy. Some of the defect mechanisms are unfavorable for lithium ion migration in the crystal structure. In order to enhance the electronic conductivity and electrochemical properties of LiMPO4 cathode materials, researchers have been playing around the microstructure of the electrode materials by carbon coating, doping with metal as well as the synthesis of nano- sized particles to obtain unfavorable defect free phase. The reported voltage ranges for LiFePO4, LiMnPO4, LiCoPO4 and LiNiPO4 are 3.4V, 4.1V, 4.8V and 5.1V vs Li/ Li+, respectively. Alkali metal oxy-phosphates AMOPO4 (A= Li & Na; M= V & Ti) are considered as a potential candidate for high energy battery applications due to their high theoretical capacity. Among all Polyanionic phosphates, tavorite-type structures are capable of very high rates with one-dimensional lithium diffusion path. Another one, AxMPO4Y Tavorite-type compounds (with A = Li, Na, Mg...; M = Ti, V, Mn, Fe; and Y = O, OH, F, and a mixture of them) are the reported for the energy storage applications. Incorporated fluoride is replacing oxides on phosphorus site of metal phosphate frameworks, which changes both the structural and chemistry of materials. Fluoride rich framework have the ability to stabilize the structure and higher oxidations states on a transition metal due to the formation of bridge between F ̶ and MO in different transition metal centers and the formation of terminal fluoride sites on [MOnF6-n]m ̶ and [PO4-pFp]q ̶ polyhedral. These fluorophosphates are exhibit high voltage and excellent energy densities with very high cycling stability [4]. Furthermore, red and black phosphorous are the most used anode materials for both lithium and sodium ion batteries after silicon. The single-layered black phosphorus, named phosphorene, has been widely studied in battery application due to the graphite-like layered structure. In phosphorene, phosphorus atom is covalently bonded with three adjacent phosphorus atoms to form a puckered honeycomb structure [5]. As per the electrochemical activity, elemental phosphorus has enough small atomic weights and great Li-uptake ability ((Li3P, 2596 mAh g−1) like silicon. The operating potential is much safer than graphite and delivers high capacity, which can be a suitable anode material for next generation alkali-metal-ion batteries.
Summary and future outlook
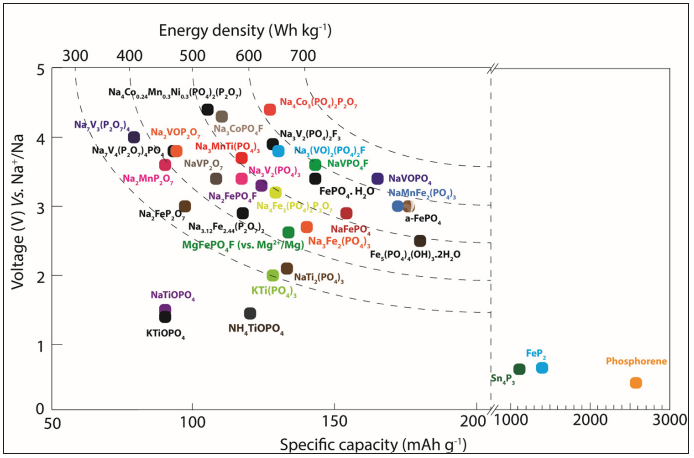
In summary, this short overview showed the amount of research efforts towards the development of phosphorus based active electrode materials for Li and Na ion batteries. Figure 1 & 2 Show the Average voltage and energy density (Wh kg−1) versus gravimetric capacity (mAh g−1) for phosphorus-based electrode materials for lithium and sodium ion batteries, respectively. The major drawback of phosphorus-based electrode materials is its poor electronic conductivity. To overcome these low intrinsic electronic conductivity and long diffusion path natures of phosphorus-based electrode materials, different strategies (Like, carbon coating, composite with reduced graphene oxide, aliovalent transition metal doping and nano size particles) were adopted successfully. Altogether, it is clear that Phosphorus based materials will bring many technological breakthroughs in the future when they are employed in next generation alkali metal ion battery applications.
Figure 1:Phosphates for Lithium ion battery applications..

Figure 2:Phosphates for Sodium ion battery applications.
References
- Fang YJ, Zhang JX, Xiao LF, Ai XP, Cao YL, et al. (2017) Phosphate framework electrode materials for sodium ion batteries. Adv Sci 4(5): 1600392.
- Huang Y, Zheng Y, Li X, Adams F, Luo W, et al. (2018) Electrode materials of sodium-ion batteries toward practical application. ACS Energy Lett 3(7): 1604-1612.
- Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho‐olivines as positive‐electrode materials for rechargeable lithium batteries. J Electrochem Soc 144 (4): 1188-1194.
- Kumar PR, Jung YH, Chek Hai L, Kim DK (2015) Na3V2O2x(PO4)2F3−2x: a stable and high-voltage cathode material for aqueous sodium-ion batteries with high energy density. J Mater Chem A 3: 6271-6275.
- Batmunkh M, Bat‐Erdene M, Shapter JG (2016) Phosphorene and phosphorene‐based materials-prospects for future applications. Adv Mater 28(39): 8586-8617.
© 2019 Amin R . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






