- Submissions

Full Text
Research & Development in Material Science
Laser Corrosion of TiN-TiB2 Ceramics
Vlasova M1*, Kakazey M1, Marquez Aguilar PA1, Guardian Tapia R1, Reséndiz- González MC1, Castro Hernandez A1, Mel’nikov IV2,3 and Ya Fironov3
1 Center of Investigation in Engineering and Applied Sciences of the Autonomous University of the State of Morelos (CIICAp–UAEMor), Mexico
2 Research Center for Marine Geophysics, Russia
3 School of Radiotechnics and Computer Technology, Russia
*Corresponding author:Vlasova MV, Center of Investigation in Engineering and Applied Sciences of the Autonomous University of the State of Morelos (CIICAp– UAEMor), Mexico
Submission: March 01, 2019Published: March 07, 2019

ISSN: 2576-8840 Volume10 Issue2
Abstract
The paper presents the physicochemical corrosion process of composite TiN - TiB2 ceramics in the mode of pulsed laser irradiation in the atmosphere. It has been established that as a result of the development of high-temperature oxidation TiN and TiB2 processes and partial dissociation of these compounds the crater on the surface is formed. Oxidation processes lead to the formation of TiNx, TiB, TiO2, B2O3 and N2, whereas dissociation yields in Ti, B and N2, correspondingly. The gas and vapor products (B2O3 and N2) and part of the TiO2 melt in the form of droplets are removed away from the crater. Such compounds as TiNx, TiB and TiO2 are enriched the surface of the crater walls.
Keywords: TiN - TiB2 ceramics; Laser pulse irradiation; Phase transformation
Introduction
Such types of mechanical treatment as cutting, drilling, scribing et al. of refractory ceramics, which are made of carbides, nitrides, and borides, are very difficult realize through the high hardness, density, and brittleness of those materials. Nonetheless, coincidence of such unique features as high melting temperatures, chemical, corrosion and radiation resistances, etc., makes it possible to exploit those ceramics as working elements in various fields of technology [1-4], where it often required shaping blanks accordingly. In order to overcome difficulties of machining, nowadays are increasingly moving to laser machining such ceramics [5-12]. Practically in all works, the main attention is paid to the morphology of the resulting surface and the temperature distribution in zone of irradiation whereas processes of underlying physics and chemistry occurring there are practically not analyzed.
The aim of this work was to study the phase transformation that happens in the composite ceramics TiN-TiB2, subjected to pulsed laser irradiation in the atmosphere. The choice of such ceramics is due to the following considerations: the individual components of ceramics (TiN and TiB2) have different melting points, dissociations, and different stability in an oxidizing medium [1,2,13,14]. The TiN- TiB2 composite ceramics also has high mechanical and friction properties, resistance to oxidation, wear resistance and so is exploited as a cutting tool [15]. Since the laser processing is the most acceptable method for such a refractory composite, it is necessary to establish the mechanism of the destruction of the ceramic target under the high-power laser action, which will make it possible in the future to manage the regime of laser processing.
Experimental Technique
The ceramics of 80 wt. % TiN - 20 wt. % TiB2 is obtained from a homogenized mixture of powders by hot pressing at 4GPa at a temperature of 1400 °C for 3min [16]. The specimen obtained have a cylindrical form with diameter d=5mm and height l=10mm. The laser processing is executed with a fiber laser model YLS-1000-SM (IPG Photonics) that operates at 1070nm and has a 1-kW average power in a gain-switched regime. The pulse width is 2ms at repetition rate 250Hz. The specimen are treated for 5 seconds in the atmosphere. The ablation products are deposited on a glass substrate located parallel to the surface of the specimen (target) at the 2cm distance. Both specimen and ablation products are investigated with the X-ray diffraction method (D2 PHASER diffractometer, Bruker) and scanning electron microscopy combined with microanalysis (were used scanning electron microscopes Hitachi SU 5000, and ZEISS-FIB-SEM).
Results and Discussion
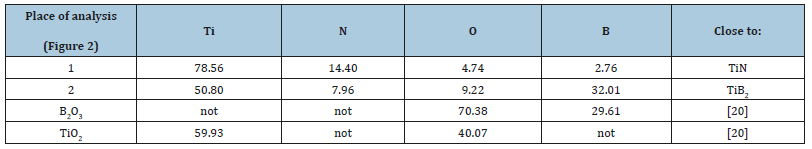
According to obtained XRD data, the main phases in ceramics are TiN and TiB2 (Figure 1a). The ceramics turned out to be a sintered grain of TiN and TiB2 (Figure 2). TiN is predominantly concentrated in bright areas, and TiB2 prevails in gray areas (Table 1).
Figure 1:X-ray diffraction patterns of ceramics obtained from 80 wt. % TiN - 20 wt. % TiB2 mixture 1(a), surface layer of track (b), and products of ablation on substrate in zone I (c).
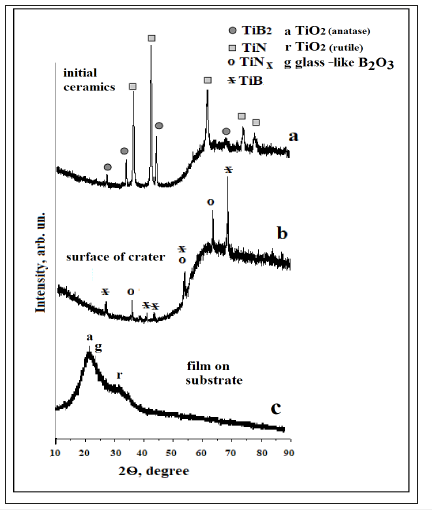
Figure 2:SEM secondary-electron micrograph of the TiN–TiB2 ceramics sintered at 1400 °C (a, b). Light gray areas correspond to aggregates of TiN grains, and dark gray areas correspond to a mixture of TiN and TiB2 grains, black holes are pores.

Table 1:Content of elements in different places of ceramic surface.
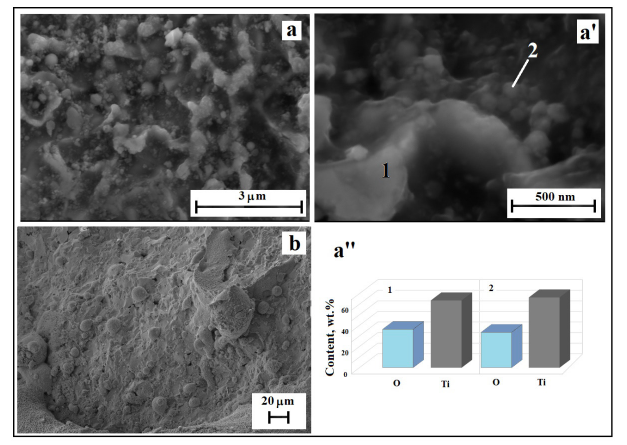
When ceramics are irradiated, a crater is formed on its surface (an analogue of laser drilling). Figure 1b reads that the phase composition of the surface layer of the crater differs significantly from the sample composition. More specifically, TiNx and TiB are fixed instead of TiN and TiB2. The surface of the crater carries signs of degradation of the composite (Figure 3a,a’). A protruding layer forms along boundaries of TiNx grains, and spherical particles are formed, too. These layers and particles contain Ti and O (Figure 3a’’). The content of elements in them is close to TiO2 (Table 1 & Figure 3a’’), what indicates the development of the processes of oxidation of ceramics, which is quite expected, since irradiation is carried out in the atmosphere.
Figure 3:SEM micrographs of crater surface (a, a’), surface of target (b) and content of elements on border of grain (1) and spherical particle (2) (a’’).
The formation of TiNx and TiB on the surface of the crater (Figure 1b) indicates the development of reactions in the irradiation zone, which are initiated by the entry of oxygen into the crater happen as soon as it is formed. Since oxygen is an active oxidizing agent, first, the reactions that occur in the crater are as follows [2,14,17-19]:
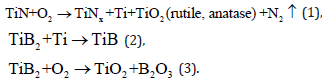
The observation of spherical particles whose composition is close to TiO2 in the crater indicates melting of the titanium oxide formed. Given the melting point of titanium oxide (Tmelt. ~ 1870 oC), it is possible to conclude the temperatures reached in the crater are ≤2000 oC. In addition, stemming from the reaction (1), a gaseous atmosphere enriched in nitrogen is created there in the crater. The excessive pressure in the crater provokes eruption of TiO2 droplets and B2O3 vapors (B2O3 at T ≤1730 oC enters the gas phase [1,20]). Due to the high volatility of boric anhydride, the range of its distribution into the surrounding space should significantly exceed the “expansion” of titanium oxide droplets when the laser pulse is switched off.
For a more complete understanding of the processes of laser corrosion of composite ceramics, one should analyze the products erupted from the crater. As can be seen from Figure 3b, products, which have the form of powder and spherical particles of various sizes (from 0.2𝜇m to 10𝜇m) are deposited on the target surface. In turn, the similar products are distributed in space and deposited across the substrate. The diffraction pattern of the film deposited on the substrate (Figure 1c) is represented mainly by the halo (amorphous phase) and weak peaks related to TiO2 in the form of rutile and anatase [21]. The complex halo can be considered as a superposition halo from anatase titanium oxide phase containing B2O3 [22] and glass-like B2O3 [23].
While examining films on a substrate formed at different distances from the source of their emission (crater), it is readily seen a significant difference in their morphology (Figure 4). Sic, a thick coating is formed in the zone I, which is the closest to the crater, and where spherical particles of various sizes are deposited (Figure 4a-a’’). In the places where spherical particles are deposited, on the coating “cracks” appear, what indicates a high rate of eruption of spherical particles from the crater. As the flight path of the resulting products increases, that is, at removing away from the crater, there is a decrease in coverage density (Figure 4b-b’’, c-c ’’). Spherical particles are observed across the entire surface of the substrate, but their size gradually decreases.
Figure 4:SEM micrographs of film surface on substrate at different distance from formed crater on target..

The microanalysis performed in the map regime shows that the titanium content gradually decreases as one moves from zone I to zone III, while the boron’s increases. The microanalysis across the area of 20x20 microns clarifies the data obtained in the map mode. As it can be seen from Table 2, the titanium content decreases and boron increases at the transition from the zone I to zone III. It should be noted that the correct assessment of boron and oxygen in the zone III is difficult due to the fact that the substrate material contains oxygen, and the ablation products form not a continuous, but as a grid coating (Figure 4c-c ’’).
Table 2:Elemental composition in different regions of substrate surface after laser treatment TiN–TiB2 ceramics.

The local analysis of spherical particles from different deposition zones (Table 1 & Figure 5) reveals the following peculiarity of changes in their elemental composition. Thus, in zones I and II, the composition of spherical particles can be attributed to titanium oxide doped with boron (TiO2: B) [24,25], and in zone III to boric oxide doped with titanium (B2O3: Ti), correspondingly. This indicates the decomposition/dissociation of TiN and TiB2 taking place in the crater zone, in parallel with the oxidation processes as well as formation of atoms and clusters of B, Ti [13], which are “captured” by the liquid drops of TiO2 or B2O3 (Table 2). Therefore, the content of dopants decreases as the particles cool down during the flight, that is, during the transition from the zone I to zone III.
Figure 5:The distribution of elements in different zones of ablation products deposition (Figure 4).
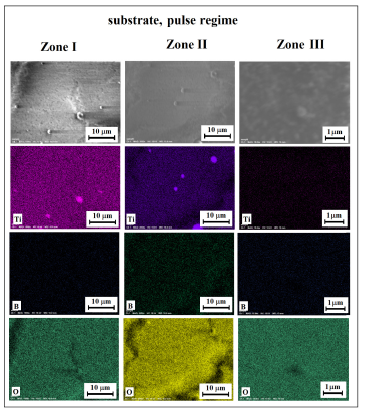
Hence, the transformation of the morphology and composition of the films depending on the “path length” of newly formed products erupting from the crater in the form of TiO2 droplets and gaseous/vapor B2O3 confirms the formation of oxides and their doping with boron or titanium, which is consistent with XRD data.
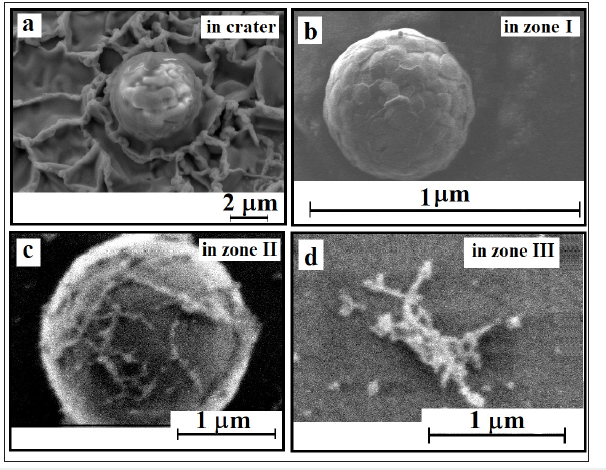
It should be noted that the correct microanalysis of the content of Ti, B, O both in spherical particles and in areas of a certain size is hard to carry out for the following reasons: drops of TiO2 and gaseous/vapor B2O3 are simultaneously erupted. As spherical particles of TiO2 cool down, boric anhydride particles are being condensed on them (Figure 6). Only in zone III, the vapor of boric anhydride is cooled and the coalescence-coagulation processes take place. In the crater itself, the cooled spherical particles TiO2 have a clean surface due to the high volatility of B2O3
Figure 6:View of spherical particles in the crater (a) and in various zones of flight (b-d).
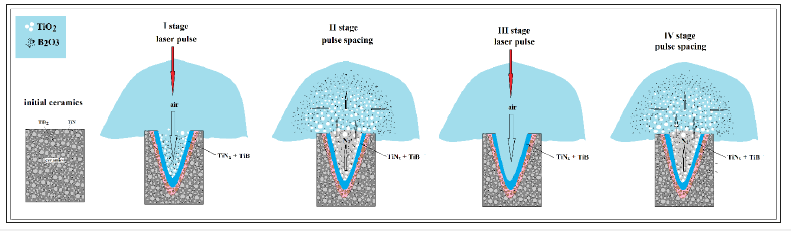
The above processes ongoing in the crater, are carried out at the duty-cycle of the laser. Between the pulses, the crater is “cleaned up” from newly formed products and while exposed to the next pulse, all high-temperature corrosion processes are launched out again as a new portion of oxygen enters the crater (Figure 7). On the one hand, this leads to the further eruption and expansion of the crater (similar to drilling) with a specific phase composition of its surface, and, on the other hand, the next bunch of laser ablation products is deposited around irradiation zone of the sample what indicates the need to airflow the focal area. In turn, the newly formed products deposited on the substrate can be the basis for the production of films. At the same time, the film deposited is a composite both in the content and in the morphology what depends on the distance from the crater.
Figure 7:X-A simplified scheme of the formation and spread of the plasma-droplet cloud from the crater.
Conclusion
Our experiments show that the pulsed laser irradiation of TiNTiB 2 ceramics leads to the formation of the crater in the sample where the surrounding air enters. The phase composition of the crater walls changes to TiNx, TiB, and TiO2, and the crater cavity itself is enriched with Ti, B, B2O3 and N2. As a result of the development of the high-temperature oxidation processes of TiN and TiB2 and the partial dissociation of these compounds the volatile B2O3, droplets of melted TiO2, as well as atoms and groups of atoms of Ti and B “erupt” from the crater under the effect of overpressure in the crater and at the termination of the laser pulse. Atoms B and Ti are doping impurities for TiO2 and B2O3, accordingly. When exposed to the next pulse, all processes are repeated again. The products of the spread of the droplet-dust cloud into the external environment can serve as the basis for obtaining coatings and films of different composition and morphology, the properties and regions of application of which are to be studied.
References
- Kosolapova TYa (1968) Carbides, Metallurgiya, Moscow.
- Serebryakova TI, Neronov VA, Peshev PD (1991) High-temperature Borides, Moscow, Metallurgia.
- Gogotsi YG, Andrievski RA (1999) Materials science of carbides, nitrides and borides, Springer Science & Business Media, Dordrecht, Netherlands.
- Matkovich VI (1977) Boron and refractory borides, Springer Verlag, New York, USA.
- Samant AN, Narendra BDNB (2009) Laser machining of structural ceramics-A review. J Europ Ceram Soc 29: 969-993.
- Hongjian W, Huatay L, Chengyong W, Lijuan Z, Xiaoyue H (2017) Laser drilling of structural ceramics-A review. J Europ Ceram Soc 37(4): 1157- 1173.
- Chryssolouris G, Anifantis N, Karagiannis S (1997) Laser assisted machining: an overview. J Manuf Sci Eng 119(4B): 766-769.
- Durand C, Ramulu M, Pierre RST, Machan J (1996) An experimental analysis of a Nd: YAG laser cutting process for machining silicon nitride, Intern. J Product Res 34(5): 1417-1428.
- Quintero F, Pou J, Lusquinos F, Boutinguiza M, Soto R, et al. (2001) Nd: YAG laser cutting of advanced ceramics, proceed. SPIE 4419: 756-760.
- Knowles MRH, Rutterford G, Karnakis D, Ferguson A (2007) Micromachining of metals, ceramics and polymers using nanosecond lasers, Intern. J Advanc Manufacturing Technol 33: 95-102.
- Ho CY, Lu JK (2003) A closed form solution for laser drilling silicon nitride and alumina ceramics. J Mater Proc Technol 140: 260-263.
- Quintero F, Pou J, Lusquinos F, Rivero F, Perez-Amor M (2007) Singlepass and multi-pass laser cutting of Si-SiC: assessment of the cut quality and microstructure in the heat affected zone. J Laser Appl 19(3): 170- 176.
- Kulikov IS (1969) Thermal dissociation of compounds, Metallurgy, Moscow.
- Voitovich RF (1998) Oxidation of carbides and nitrides, Kiev, Naukova Dumka, Ukraine.
- Petuhov AS, Hobta IV, Ragulya AV (2012) Prospects for the development of cutting material based on the compositions TiB2-TiN. Series: Physical and chemical fundamentals of powder materials technology 21: 186- 199.
- Bykov A, Vlasova M, Márquez-Aguilar PA, Kakazey M (2016) Obtaining at high pressure the TiN-TiB2 ceramic nano-composite. Mater Sci and Appl 7: 232-237.
- The Physics and Chemistry of Carbides, Nitrides and Borides (1989) Freer R (Ed.), Kluwer Acad. Publ., Dordrecht, Netherlands.
- Koh YH, Lee SY, Kim HE (2001) Oxidation behavior of titanium boride at elevated temperatures. J Am Ceram Soc 84(1): 139-141.
- Andrievskii RA, Shul’ga YM, Volkova LS, Korobov II, Dremova NN, et al. (2016) Oxidation behavior of TiB micro- and nanoparticles. Inorgan Mater 52(7): 686-693.
- The oxide handbook (1973) Samsonov GV (Ed.), IFI/Plenum data Corporation.
- Kenji S, Mari M (2010) X-ray diffraction imaging of anatase and rutile. Anal Chem 82(9): 3519-3522.
- Saidi W, Hfaidh N, Rasheed M, Girtan M, Megriche A, et al. (2016) Effect of B2O3 addition on optical and structural properties of TiO2 as a new blocking layer for multiple dye sensitive solar cell application (DSSC). RSC Adv 6: 68819-68826.
- Can AS, Öztürk A, Kalem V, Park J (2016) Influence of TiO2 Content on the Photocatayltic Activity of TiO2-B2O3 glasses prepared by the sol-gel process, IMMC 2016. 18th Intern. Metallurgy and Materials Congress, pp. 40-43.
- Deependra DM, Thapa D, Dahal B, Baral D, Solanki PR (2016) Spectroscopic studies of boron doped titanium dioxide nanoparticles. Intern J Mater Sci Engineer 4(3): 172-178.
- Artiglia L, Lazzari D, Agnoli S, Rizzi GA, Granozzi G (2013) Searching for the formation of Ti-B bonds in B-doped TiO2-rutile. J Phys Chem C 117(25): 13163-13172.
© 2019 Vlasova M . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






