- Submissions

Full Text
Research & Development in Material Science
Focculation of Paper Mill Effluents Using Starch-GPolyacrylamide Flocculants
Karmakar GP1* and Singh RP2
1Department of Mining Engineering, Indian Institute of Technology, India
2Indian Institute of Science Education and Research, India
*Corresponding author: Karmakar GP, Department of Mining Engineering, Kharagpur 721302, India
Submission: October 26, 2018; Published: October 29, 2018

ISSN: 2576-8840 Volume8 Issue4
Abstract
Co-polymers of potato starch and acrylamide have been synthesized using ceric-ion-initiated solution polymerization technique Intrinsic viscosities.elemental analysis, XRD and thermal analysis have been carried out. analysis, The synthetic polymers have been used as flocculants for the treatment of effluent collected from Emami Paper Mills, Balasore, Orissa, India. For the above effluent both coagulation and flocculation studies have been carried out using various coagulants and the synthetic flocculant. The effects of various coagulants in combination with the synthetic flocculant and their concentrations on flocculation have been studied. The supernatant turbidity of the coagulated/flocculated effluent has been measured for each concentration of the effluent. A wide range of concentrations of the effluent has been chosen to predict the appropriate dilution required for the flocculation of such systems. The flocculation efficiency of the synthesized polymer has been compared using Magnafloc 1011 (M/s Allied Colloids, UK). It has been observed that due to the complex nature of the paper mill effluents, coagulation with electrolytes is essential before the flocculation of such systems with the synthesized polymer and the synthesized polymer may be used as flocculation aid for such systems.
AbbreviationsPPM: Parts Per Million; RPM: Rotations Per Minutet
Introduction
Starch and alum have been widely used as flocculants in various industries. The biodegradability of starch molecules and the increasing of the ionic contents by the alums have ultimately led to almost discontinue the use of starch and alums as flocculants/ coagulants. Recently the high molecular weight polyacrylamides have been widely used as polymeric flocculants in solid-liquid separations in many industries. Thus the use of water-soluble polyacrylamides as flocculants has drawn much interest in recent times. The shear degradability of polyacrylamides caused concern for its use at high shear rates when polyacrylamide chains are being broken producing less effective flocculants. Deshmukh et al. [1] have synthesized polymeric flocculants based on natural polysaccharides in their laboratory. It has been found that these grafted polymers can replace such traditional polymers based on polyacrylamides and hence, reduces our dependence on petrochemically derived monomers. In this work, investigation has been carried out on grafting acrylamide monomers on starch backbone in laboratory scale and has been scaled up to 10litre capacity batch reactor level. Flocculation studies have been carried out using the synthesized polymeric flocculant alongwith various coagulants.
Experimental
Polymer synthesis
Synthetic copolymers of potato starch and acrylamide have been prepared using ceric ion initiated solution polymerization technique. The experimental details have been reported elsewhere [1-3]. The laboratory scale synthesis has been scaled up to 10litre batch reactor level [4]. The synthesized polymer has been dried at 40oC under vacuum till the constant weights have been observed for the last two readings. The dried product has been pulverized and has been used for flocculation studies.
Flocculation studies
Coagulation/flocculation studies have been carried out using a laboratory jar test apparatus known as flocculator (M.B.Flocculator; M.B.Instruments, Mumbai, India). The supernatant turbidity of the flocculated effluent has been measured for each concentration of the effluent using a Systronics Digital Nephelo- Turbidity Meter 132(M/s Systronics, Ahmedabad, India). The turbidity of the supernatant liquid for each case has been determined in standard Nephelometric Turbidity Units (NTU). A wide range of concentration of the effluent has been chosen to predict the appropriate dilution required for the coagulation/flocculation of such systems. The results have been compared with those obtained using Magnafloc-1011, a commercial anionic flocculant.
Results and Discussion
Figure 1 shows the coagulation-flocculation after 10times dilution of the effluent. The diluted effluent has been first coagulated with Al2(so4)3.14 H2O by fast mixing at 50rpm for 2 minutes. Subsequently it has been subjected to slow mixing for 10 minutes and settling for 30 minutes. The 10ppm concentration of polymer SAM-S-II (Starch Grafted Polyacrylamide Scale up-II) has been fixed while varying the alum concentrations from 50ppm to 2250ppm. It is observed that as the alum concentration increases, the turbidity of the supernatant liquid increases from 27.5 NTU at 50ppm to nearly 50NTU at 1250ppm of alum concentration. When the alum concentration is increased further, bigger size of floc formation is observed at 1500ppm onwards and the turbidity of the supernatant liquid is gradually lowered to 7.8NTU at 2250 ppm of alum concentration. Next, the effluent sample has been further diluted to 20times with water and then has been coagulated and flocculated by addition of the same alum and polymer respectively, by standard jar test method. The variation of the supernatant turbidity for various coagulants and flocculants has been plotted in Figure 2. It is observed that after an initial destabilization with 50ppm concentration of alum, the increase in alum concentration causes restabilization of the fines and the supernatant turbidity maximum of 70 NTU is obtained in 350ppm concentration of alum. Thereafter, suddenly the supernatant turbidity falls with the addition of more alum and reaches 5.7 NTU at 1000ppm concentration of alum. It clearly indicates that the dilution of the effluent helps the coagulation of the colloidal system by generating new colloidal systems. Although the polymer concentration of 10ppm remains constant for both 10times and 20times dilution, it can be seen that total quantity of required coagulant for diluted system is less than the concentrated system alone (at 10times dilution, 2250ppm can lower the turbidity to 7.8 NTU and at 20 times dilution, 1000x2ppm=2000ppm reduces the turbidity to 5.7 NTU).
Figure 1:Variation of supernatant turbidity with changing alum doses for paper mill effluents
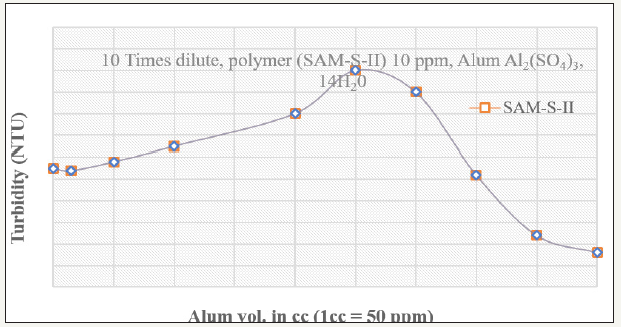
Figure 2:Variation of supernatant turbidity with changing alum doses and polymer for paper mill effluents
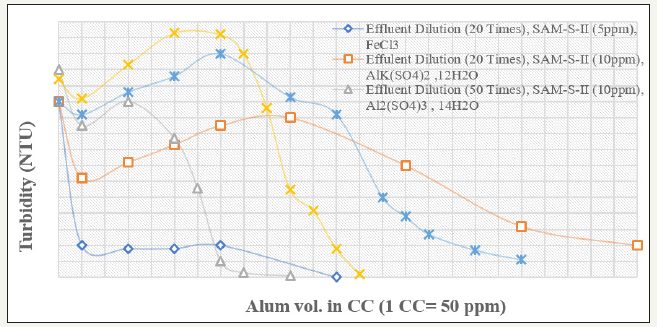
The effect of various coagulants has been studied for 20times diluted effluent. The flocculation efficiency of the SAM-S-II after coagulation is shown in Figure 3 & 4. It can be seen that for this particular effluent, Alk(so4) 12H2O is better coagulant than Al2(so4)3. 14H2O and FeCl3) is the best among these three coagulants. When FeCl3) is being used keeping fixed dose of 5ppm of SAM-S-II, the turbidity value of the 20 times diluted effluent is lowered to 9.7 NTU with addition to 50ppm of FeCl3). This is further lowered to 0.5 NTU at 600ppm of FeCl3). When compared with Magnafloc-1011, it is observed that, at the lower coagulant dose range (50ppm to 750ppm), the ability to flocculate the coagulated particles by Magnafloc-1011 is better than SAM-S-II. However, beyond 350ppm dose, increase in coagulant concentration results in sudden fall in the supernatant turbidity to 0.5 NTU by SAM-SII at 600ppm (6.0 NTU by Magnafloc-1011) indicating almost total flocculation of the coagulated particles. Thus when the complex paper industry effluent is diluted and coagulated with FeCl3), and then flocculated with the starch-g-copolymer, the supernatant turbidity can be lowered to an acceptable value of 0.5 NTU (Figure 4). The concentrated effluent solutions (dilution less than 10 times) could not be treated effectively. This is due to the fact that paper industry effluent mainly contains lignosulfonates, lignin, oligo-and polysaaccharides and other high molecular weight colour forming compounds. Even after dilution up to 10times with water, it is found to be very difficult to flocculate the particles with polymeric flocculants only. The coagulation of the colloidal system is necessary with metal coagulants like alum or ferric chloride prior to flocculation. The solubles are generally anionic in character and can be expected to react with added coagulants to generate new colloidal components in the system, as well as to reduce the dose of coagulant/flocculant [5]. Again at high dilution, the initial turbidity is increased due to presence of the solubles. For the clarification of the system of fines alongwith the solubles both coagulants and flocculants are needed.
Figure 3:Variation of supernatant turbidity with various coagulants and their varying doses for paper mill effluents
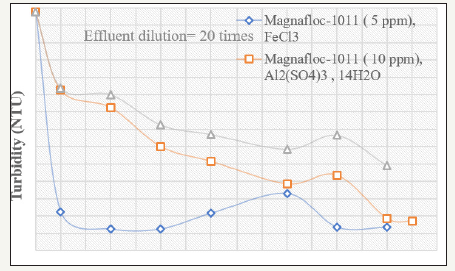
Figure 4:Variation of supernatant turbidity with various FeCl3) doses using various polymers for paper mill effluents
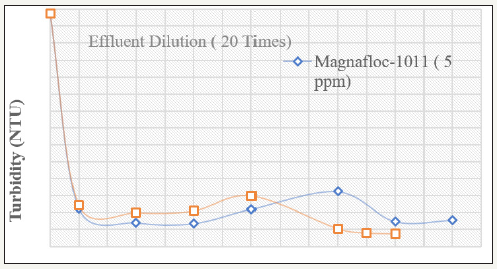
The major control being the electrostatic or charge neutralization, the coagulation must be carried out prior to flocculation by bridging the flocculated particles in the paper industry effluents. The ferric chloride is better coagulant than the alum. This is due to the complicated hydrolysis reaction of aluminium salt solutions that is responsible for the narrower pH range often evident for optimum destabilization when compared with iron solution. With iron salts, adsorption follows a Langmuir adsorption isotherm, whereas with aluminium, adsorption often follows a Freundlich isotherm. This is a further indication of complicated hydrolysis products formed by aluminium salts [6]. The initial pH of the effluent is 6.9 and after dilution also, the pH remained almost constant. When alum is added, the pH becomes slowly acidic; but treating with only polymer, no change in pH of the system is observed. This is due to the increase in Al+++ ions in the system during the addition of alum. The same trend of increase in pH is observed with FeCl3) treatment due to the increase in Fe+++ ions in the system. Besides the above, an account of more detailed studies on polysaccharide based grafted copolymers and their flocculation characteristics have been reported elsewhere by the author and his co-workers [7,8].
Conclusion
A. While treating the effluents from paper mills it is observed that neither a coagulant nor a flocculant can completely flocculate such complex systems. After destabilizing such effluent system with a suitable coagulant, starch grafted copolymer can be used as a flocculant aid for the complete flocculation of the contaminants present in the system.
B. Ferric chloride is better coagulant than alums used in the above-discussed system.
The synthesized starch-g-polyacrylamide has shown effectiveness at par with the Magnafloc-1011 while using very small quantity of the flocculant.
References
- Deshmukh SR, Singh RP (1987) Drag reduction effectiveness, shear stability and biodegradation resistance of guar gum-based graft copolymers. J Appl Polym Sci 33(6): 1963-1975.
- Ungecheur S, Bewersdorff HW, Singh RP (1989) Turbulent drag effectiveness and shear stability of xanthan gum-based graft copolymers. J Appl Polym Sci 37(10): 2933-2948.
- Deshmukh SR, Sudhakar K, Singh RP (1991) Drag reduction efficiency shear stability and biodegradation resistance of carboxymethyl cellulose- based and starch-based graft copolymers. J Appl Polym Sci 43(6): 1091-1101.
- Karmakar GP, Singh RP (1993) Scale up of laboratory synthesis of starch-acrylamide and its application as flocculant, national workshop on ceramics, polymers and composites, NML, Jamshedpur, April 23-24.
- Donnan MB, Healy TW, Nelson PF (1981) An electrokinetic study of alum coagulation and polymer flocculation of cellulose pulp fines. Colloids and Surfaces 2(2): 133-143.
- Bartby J (1980) Coagulation and flocculaltion with an emphasis on water and wastewater treatment, uplands press ltd. Croydon CR 9 1LB, pp. 1-376.
- Singh RP, Tripathy T, Karmakar GP, Rath SK, Karmakar NC, et al. (2000) Novel biodegradable flocculants based on polysaccharides. Current Science 78(7): 798-803.
- Karmakar GP (2017) Synthesis, characterization and flocculation characteristics of polysaccharide graft copolymers. Res Dev Material Sci 1(4): 1-2.
© 2018 Oleg L Figovsky . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






