- Submissions

Full Text
Research & Development in Material Science
Biosorption of Fluoride from Water by Fabricated Chitosan Doped Graphite Novel Composite
Rajendra S Dongre*
Department of Chemistry, RTM Nagpur University, India
*Corresponding author: Rajendra S Dongre, Department of Chemistry, RTM Nagpur University, Amravati Road, Ram Nagar, Nagpur, Maharashtra, India
Submission: June 01, 2018;Published: July 10, 2018

ISSN: 2576-8840 Volume7 Issue1
Abstract
Defluoridation/fluoride removal from water is achieved by fabricated chitosan doped graphite novel composite (FCDGNC). Biosorption is widely used due to economic, operative and maximum efficiency, more recyclable competence and ecological benign advantages. Many adsorbents reported for defluoridation below 2ppm had displayed low/moderate capacity besides, operated at drastic pH. So, batch de-fluoridation aimed to perform with respect to parameters like pH, dose, contact time and fluoride concentration. Water defluoridation was highly pH sensitive and found optimum pH=6.5 and sorption thermodynamics confirmed endothermic behavior besides chemi/physisorption. Kinetics followed pseudo 2ndorder while fluoride adsorption equilibrium wereanalyzed for Langmuir and Freundlich, models, but better suited to Langmuirwith adsorption capacity of 37.9mg/g. FCDGNC were regenerated and reuses better upon five defluoridation cycles to achieved fluoride stringent limit 1.5-2ppm without affecting quality of treated water. Thus, FCDGNC intended for efficient and most convenient way proposed further for large scale utility.
Keywords:Biosorption;Chitosan;Biocomposites;Defluoridation;Water
Highlights
a. Fabricated chitosan doped graphite novel composite (FCDGNC) is easy to prepare.
b.The FCDGNC powder form owes efficient fluoride sorption capacity.
c. The FCDGNC owes very efficient adsorption capacity for fluoride reach 3.79mg/g
d. The FCDGNC biosorbent can be easily regenerated and reused in five defluoridation cycles.
Introduction
Biosorption is an endowed technique practiced for removal of organics, inorganics, and metal contaminations from water Fomina & Gadd [1]. For sustainable environment, biosorption offers advantages as its cost-effective, environmentally friendly and virtually unlimited supply of biopolymers/products resources Wang & Chen [2]. In our planet, diversified bio-adsorbents exist, ranging from micro-organisms to agricultural waste Babel & Kurniawan [3] for this purpose. Ubiquitous, chitin, is the most talented biopolymer exist after cellulose in nature which is acetylated linear polysaccharide of β-(1,4)-D-glucosamine and chitosan is deacetylated-chitin/oligosaccharides of 2 to 20 units similar to cellulose being vital components in exoskeleton of crustacean, fungi and insects Sashiwa & Aiba [4]. Nevertheless, chitosan suffers due to poor mechanical properties and low chemical resistance Barber & Rogers [5] that can be improved by means of impregnation with materials like ceramic-alumina Liu [6], Madala & Abburi, alginate Ngah & Fatinathan, polyvinylalcohol Zhu & Zeng [7], cyclodextrins Liu & Economy [8], magnetic nanoparticles Reddy DH & Lee SM [9], ionic liquids Cui & Men [10], and silica Witoon & Limtrakul [11]. Both glucosamine biopolymers impart non-toxicity, biodegradability and reusability advantageous by means of grafting ample acetamido/amino groups to amend composite’s skeleton exhibited high adsorption capacity/selectivity for pollutants Barber & Rogers 2014, Gupta & Suhas [12].
Fluoride is ubiquitous and enters in our body through water, food, industrial exposure, drugs and cosmetics etc; nevertheless, drinking water is the single major source of its daily intake WHO [13]. Fluoride in water below 1.5ppm aids in prevention of tooth decay, besides assisted in bone development, but if present above/heavy dose over long span causes dental, skeletal/nonskeletal fluorosis, tooth mottling, osteoporosis, collapsed vertebrae, and accumulative toxins Viswanathan & Siva Ilango [14]. Worldwide ground water is used for drinking purpose and fluoride contaminations in such waters are mainly due to leaching from ores, minerals/parent rock or artificially via industrial wastewaters besides contaminated surface waters Arora & Chattopadhya (1974). International Agency for Research on Cancer (IARC, 2007-08) has determined that fluoride carcinogenicity isn’t classifiable, so prior consumptions, its removal i.e. de-fluoridation is the best and only form of treatment of fluorosis and above all calamities.
De-fluoridation techniques are based on the phenomenon of chemical precipitation, adsorption, ion exchange, electrolytic and electro-dialysis Turner & Stipp [15]. And, amid precipitation, adsorption/bio-sorptions are exhaustively used in defluoridation treatment for water Davila & Rodrigue [16]. In general, precipitation involved treatment with iron, aluminum, calcium, and lanthanum salts forming insoluble precipitate seems convenient and economical, but owes limited removal capacity (precipitated fluoride gets soluble), auxiliary huge toxic sludge generation Gogate & Pandit [17]. Consequently, adsorption is preferred due to benign and efficient viability on field; yet, adsorbent’s cost restricted its viable industrial utility besides environmental norms urge to explore promising bio-sorbents Qu [18]. Literature revealed usages of varied conventional and non-conventional adsorbents for fluoride removal viz; oxides/hydroxides, biosorbents, geomaterials, carbonaceous and industrial products/by-products Crini [19]. Nevertheless, especial fabricated chitosan bio-sorbents exhibited excellent defluoridation with higher efficiency over other synthetic adsorbents Pandi [20], Swain [21] and Viswanathan & Meenakshi, [22].
In this contest, chitosan derivatives and modified forms are most frequently utilized for defluoridation, though pure chitosan flakes/powder is seldom used due to less solubility and stability that causes significant pressure drop and affects filtration to outweigh its biodegradable advantages Knaebel & Hill [23]. Chitosan’s flexible skeleton can be modified by doping with inorganic materials like zeolites, clays and minerals to yield synthetic bio-composites for mitigation of fluoride contaminations Pandi [20] and Swain [21] and Viswanathan & Meenakshi [22] and Li & Zhu [24]. Certain literature reported use of graphene, i.e., one atom thickness planar carbon sheets for defluoridation of water, with moderate fluoride removal capacity Li & Zhu [24]. Unequivocally, these chitosan modified composite are superior to conventional adsorbents, due to unique benefits like unaffected uptake viability; weak physicochemical parameters can be overcome by articulation of -NH2/OH linkages responsible for biosorption, complextion, and/ or diffusion in their accessible pore size. In this contest, graphite’s hexagonal layers are doped on porous chitosan, yields novel biocomposite to cater above anticipated defluoridation challenges. Kinetics of FCDGNC govern bio-sorption of fluoride found as rapid, indicated its possibility of a practicable operation in packed column scale for water treatments.
Materials and Methods
Materials and apparatus
All the chemicals used were either AnalaR, GR, high purity grade and used without further purification. Chitin was purchased from M/s BR Corporation, Mumbai, as coarse grains and graphite from Loba Chemie, Mumbai (India). Acetic acid (99.5% Merck) and ammonium hydroxide (30% Merck) solutions were used. Rotary Shaker (Remi), Digital pH meter (Hanna) used and pH meter standardized by buffer pH 4 and pH 9 (Fisher Scientific,). The pH adjustment was done by 0.1N HCl/NaOH (Fisher scientific). Stock solutions containing 1000mg/L of fluoride prepared from NaF salt dissolved in distilled water and working samples prepared from stock by dilution. The batch studies attempted with synthetic water and mixtures agitated on shaker. Duplicate measurements also conducted to assure reproducibility of residual concentration within ±2%. All the solutions were made by using double distilled water. Residual fluoride was analyzed by ion selective electrode using Orion Ion electrode instrument Witchford, UK. Blank (distilled water) also run to investigate possibility of any contributions.
Synthesis of fabricated chitosan doped graphite novel composite (FCDGNC)
Figure 1:
1a: FTIR Of pure Graphite,
1b: Chitosan,
1c: FCDGNC-BT: Graphite doped chitosan before fluoride sorption and
1d: FCDGNC-AT: Graphite doped chitosan after fluoride sorption.
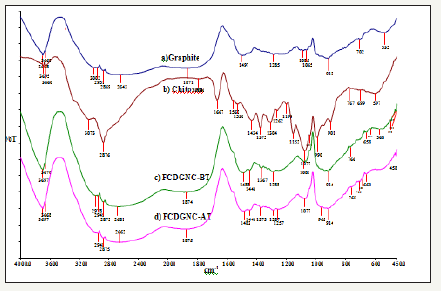
FCDGNC was synthesized by impregnation method; about 1g in 100ml 2% acetic acid chitosan (80% deacetylated) solution was stirred with graphite powder (20% w/w) on a magnetic stirrer for 5-6 hrs at agitation speed of 500rpm at NTP. The resultant syrupy mixture was sprayed by syringe in 50% aqueous ammonia to obtain hydrogels/beads Figure 1a-1c. Finally, beads filtered, washed with distilled water to remove dirt, particulate matter or any color and dried in oven at 90-105 °C for 24hrs. The FCDGNC beads were grounded in mortar pestle and sieved to get particle size of 175μm- 245 μm (stored in PVC bottles) and kept in desiccators so as to use for biosorption Figure 2a-2c.
Figure 2:
1a: FTIR Of pure Graphite,
1b: Chitosan,
1c: FCDGNC-BT: Graphite doped chitosan before fluoride sorption and
1d: FCDGNC-AT: Graphite doped chitosan after fluoride sorption.

Adsorption experiments
About 100ml of 10ppm fluoride sample was taken in a PVC bottles and known weight of FCDGNC introduced and flask was then kept on a rotary shaker for 24hrs to attain equilibrium. After completion of adsorption, solution was filtered through Whatman filter paper no.42 and filtrate was analyzed for residual fluoride concentration by ion selective electrode. The defluoridation was performed at NTP in batch mode. The effect of various parameters like bio-adsorbent dose, initial fluoride concentration, presence of interfering ions and pH on defluoridation capacity were studied Viswanathan & Meenakshi, [22].
Characterizations
Table 1:Characteristics of FCDGNC novel composite, pure chitosan and pure graphite.
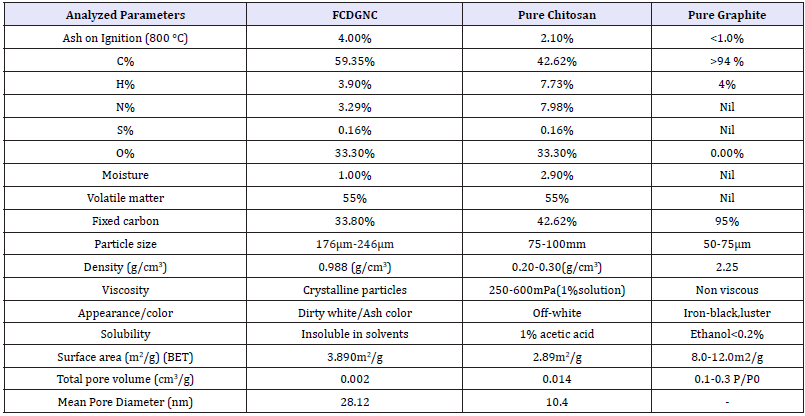
Elementary analysis: (viz; C/H/N/S/O, % Ash, %Moisture, Surface parameters) The elementary analysis shown in Table 1, Ash content calculated by Eqn (1), % moisture by gravimetry and water mass/weight difference of wet-dried sample and calculated by Equation (2)
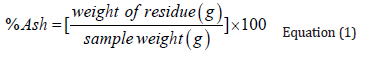
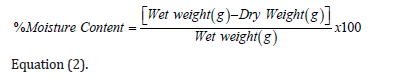
FTIR analysis: The pure chitosan, graphite and FCDGNC FTIR before and after defluoridation were presented in (Figure 3). Chitosan showed characteristics FTIR at 3695cm-1, 3073cm-1 and broad band at 2800-2950cm-1 due to stretching vibration of –OH El Hefian & Yahaya [25]. FTIR at 1667cm-1 for -C=O stretch in amide and at 1152cm-1 for bridge-O-stretching. Methylene and methyl bending vibrations in FTIR found at 1375cm-1 and 1434cm-1 respectively and FTIR at 1262cm-1 credited for C-O-H stretching and at 1077cm- 1 ascribed to C-O stretching vibration of C-O-H, C-O-C and CH2OH in ring skeleton E.de Souza Costa et al. 2009; Krishna Rao et al. 2006). FTIR at 1667cm-1 for C=O stretch in amide disappeared in FCDGNC due to graphite doping and FTIR at 1375cm-1 remarkably found at 1367cm-1 for C-H stretch attributed to physical blends/chemical interactions El Hefian [25]. The good doping graphite in chitosan matrix in FCDGNC before and after defluoridation exhibited either decreases in FTIR intensity or disappears. FTIR band of FCDGNC shows some shift in wavenumbers like 2872cm-1 band little shifted to 2875cm-1 Figure 3a-3d, suggested participation of –OH in trapping fluoride. The weak broad FTIR band at 2681cm−1 shifted to 2662cm−1 besides change observed at 1081cm-1 to 1077cm-1 due to C-O stretching vibrations suggested the involvement of C-O group in binding of fluoride on composite Habuda Stanić & Flanagan [26]. All these absorption band shifts showed fluoride sorption by C-O and amino/hydroxyl groups of chitosan that gets protonated to NH+3/OH+2 < pH 6.5 capably sorbed anions via charge neutralization.
Figure 3:SEM images of
3a: FCDGNC at 1500X,
3b: FCDGNC composite at 4000X,
3c: Graphite at 250X
3d: Chitosan skeleton at 5000X.

Thermo gravimetric analysis (TGA): Thermo-gravimetric analysis is used to determine decomposition temperature of materials Witoon & Limtrakul [11]. FCDGNC composite exhibited 1st decomposition at 38 °C continued until 200 °C with 5% weight loss due to evaporation of water and 2nd decomposition at 265.15 °C and continued to 321.6 °C with 18.37% weight chitosan loss and maximum degradation at 288.55 °C which ends at 954.9 °C with total 35% weight loss of chitosan skeleton Viswanathan & Meenakshi [22]. Nevertheless, two exothermic peaks of FCDGNC namely at 292 °C and 270.89 °C respectively shown in Figure 2c indicated less thermal stability of FCDGNC composite than pure graphite Figure 2b, instead more stable than pure chitosan Figure 2c.
Scanning electron microscope (SEM) analysis: SEM exhibited visual confirmation of physical state and surface morphology, porosity and particle size dimensions of graphite, chitosan and FCDGNC. SEM at magnifications showed chitosan’s spherical microparticles owes wrinkled surface and nonporous, uneven granular structure Figure 3c. SEM of graphite exhibited individual needle shaped particles with intrinsic flake morphology Figure 3d. SEM of FCDGNC exhibited changes in crystal agglomerated with size range 175μm to 246 μm (pore volume 28.12nm) and irregular surfaces/rugosity due to cross-linking/fractures, different than non-homogenous chitosan Figure 3a & 3b. The FCDGNC entirely differs from chitosan and graphite, due to doping of supported graphite reinforcement to alter surface properties of chitosan. These smooth voids/cavities imparts good surface area for fluoride sorption Viswanathan & Meenakshi [21].
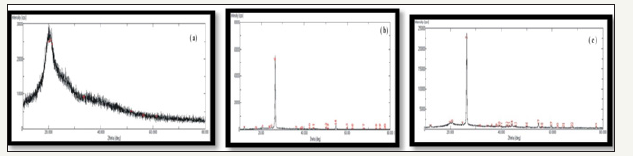
XRD analysis: XRD of chitosan, graphite and FCDGNC illustrated in Figure 4a-4c respectively. XRD of chitosan exhibited broad diffraction peak at 2Ø=10°, 20.2° and 20.74° with d spacing 4.2Å as characteristic fingerprints of semi crystallinity with no impurity peaks Alagumuthu G & Veeraputhiran V [27]. FCDGNC showed a diffraction peak at 2Ø=26.5° with d- spacing 3.35Å, due to graphite reinforcement indicated single phase composition. While FCDGNC showed broad peak at 2Ø=20° due to decreased in intensity after doping with graphite and confirms reinforcement in chitosan. Broaden small peaks around 2Ø=18° to 22° and a few peaks at 2Ø=40° and 44° in FCDGNC showed successful graphite doping in chitosan providing an auxiliary surface support along with good crystallinity
Figure 4:Powder XRD pattern of
4a: Chitosan
4b: Graphite
4c: FCDGNC composite.
Result and Discussion
Effect of graphite doping
The effect of graphite doping on chitosan for removal of fluoride from water were studied from range of 5% to 30% (w/w). It was observed that 20% of graphite doping is optimum and higher graphite doping induced leaching from FCDGNC skeleton besides the permissible fluoride limit in water gets achieved with mere 20% graphite loading.
Effect of FCDGNC composite dose
Figure 5:Adsorption Mechanism of Fluoride onto FCDGNC.
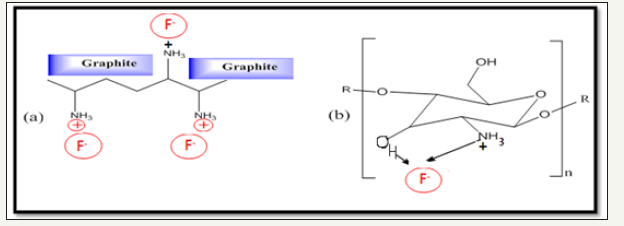
FCDGNC dose affected adsorption at fixed initial fluoride concentration as shown in Figure 4a-4c. As absorbent dose enhanced, the fluoride removal found increased but, loading capacity (fluoride loaded per unit weight of FCDGNC) found gradually decreased Liang P & Zhang Y [28]. The maximum de-fluoridation capacity was 3.5mg/g for 2.5g/l of FCDGNC dose as shown in (Figure 5). Initially, higher fluoride adsorbed per unit mass of adsorbent (due to more fluoride/adsorbent ratio) which continued to increase up to dose of 10g/l of FCDGNC. Further increase in FCDGNC doses not showed appreciable progress in defluoridation relatively down at lower adsorbate/fluoride ratio (caused negligible sorption).
Effect of initial fluoride concentration
The effect of initial fluoride concentration on % fluoride removal was studied at different range of varying fluoride concentration, by keeping other parameters constant. The adsorption revealed that if fluoride initial concentration increased, then defluoridation found decreased as attributed to saturation in binding capacity of FCDGNC (higher fluoride level later decreased % fluoride removal).
Effects of pH
The pH of medium is vital factor that affects defluoridation capacity of FCDGNC, so adsorptions were performed in range of pH 5 to pH 9. The maximum fluoride adsorption capacity found at pH 6.5. However, in alkaline medium (i.e. pH>7.5) de-fluoridation capacity found decreased due to electrostatic repulsion faced by fluoride by negatively charged surface FCDGNC (competition for active sites by excessive amount of hydroxide anions). At extreme acidic pH< 5, FCDGNC gets unsuitable/insignificantly for fluoride removal due to protonation of OH and NH2 to hydronium/H3O+ and ammonium/NH+4 respectively can dactivates binding sites of FCDGNC. In above defluoridation study pH 6.7 seems optimum.
Effects of contact time
The contact time affected delfuoridation by means of FCDGNC which followed linearly over the span of three hours achieved 75 to 80% fluoride sorption in 180min. It was observed that fluoride removal increased upto 200min with 91% removal efficiency and later found not substantial as it established equilibrium. The fluoride sorption found controlled by active binding sites of FCDGNC and accordingly equilibrium/contact time sets to 200min to facilitate proper contact and to promote effective fluoride transport in solution. Initially, large amount of binding sites of FCDGNC were available for fluoride sorption, but gradually with equilibrium, they gets worn-out and fluoride deported from exterior to interior.
Effect of agitation speed
In adsorptions agitation speed is vitally affecting the external boundary film and the distribution of adsorbate/fluoride in the bulk solution. Hence, agitation speed was varied in range of 50- 300rpm for adsorption experiments. Initially, fluoride adsorption was lower at 50, 100 and 150, but enhanced at 180 and remains steady >200rpm (so kept optimal speed) attributed to little boundary layer resistance besides high mobility.
Regeneration of FCDGNC composite
Regeneration/desorption for FCDGNC was performed at pH=8 after every cycle of defluoridation. Initial electrostatic binding of fluoride onto amino/hydroxyl of FCDGNC gets weakened drastically in extreme basic pH >8 due to competition with OH. Literature, supports decreased fluoride removal at pH >7 due to inability of proactive sites of composite. It’s known that at basic conditions (pH>7) chemisorptions decreases, due to deprotonation of since chitosan surface resulting in less sorption. Ngah & Fatinathan [29] Thus, FCDGNC can be reused in 5 cycles of delfuoridations with mere 44% reduction in its capacity.
Effect of other impurities/co-anions
Water generally contained anions like sulfate, phosphate, chloride carbonate and bicarbonate which also get participated with fluoride if present substantially in adsorptions. Hence, interfering anions affecting adsorption were studied with 0.1M salt solutions of chloride, sulfate, carbonate and bicarbonate, separately. The% fluoride adsorption remains almost constant up to a 30mg/L of sulfate concentration, thereafter defluoridation decreased and finally found 15.44% for 100mg/L sulfate interference. However, defluoridation decreased if phosphate level was 10mg/L that further decreased to 24.34% for 50mg/L phosphate interference. Thus defluoridation due to impurity followed the order of phosphate< sulfate< carbonate as they changed pH as well as competing soprtions. The higher charge density/multi-charge anions preferred over monovalent fluoride as shown in (Figure 6). The pH of fluoride solution were 8, 7 and 9, respectively, for Cl-, HCO3- & CO3 -interefering anions while pH was 6.5 without interfering impurity.
Figure 6:Preparation scheme for chitosan gels/bid of FCDGNC (scale bar=5mm).
Adsorption mechanism of fluoride on FCDGNC
The proponents of fluoride adsorption argued its economical efficiency for fluoride sorption occurred in following phases:
(1) F diffuses to adsorbent surface from bulk across boundary layer via external mass transfer;
(2) Adsorption of fluoride on to particle surfaces of adsorbent;
(3) Sorbed fluoride exchange with structural elements inside adsorbent and transferred to internal surfaces for porous via intra particle diffusion.
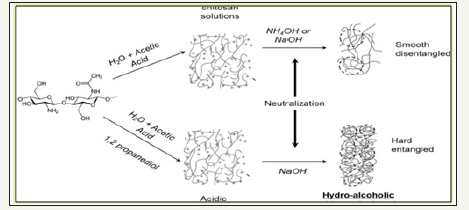
Preparation scheme for chitosan gels/bid of FCDGNC
At first, chitosan obtained as ionically cross-linked/intertwined hydrogels by dissolving in aqueous acetic acid and this pH inversion collision affected hydrophilic and phobic interaction to induce spontaneous entanglement of chitosan network to self-standing microsphere gel with chitosan content 2 to 3% as dispersed in 95 % water Swain & Airoldi [11]..This followed by coagulation in an alkaline solution which subsequently formed viscous droplets/ bids which were treated with graphite residue to yield desired FCDGNC powder after drying and proper meshed/crushing. While chitosan’s gel drying via evaporation causes dramatic shrinkage with continuity in pore size ranging from large mesopores to macropores and lose its porosity owing moderate specific surface area. This macroporosity of developed bids is attributed to space zones of contacts between chitosan fibrils though impregnation on amino groups (NH2) by dopents Reddy & Lee [9].
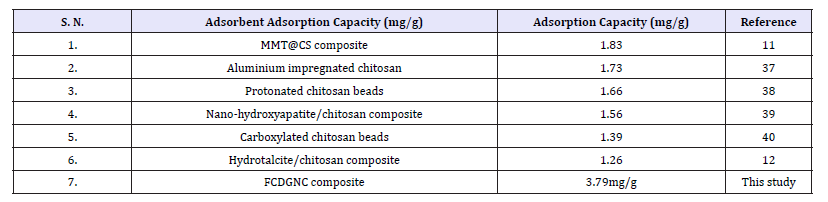
Overall, fluoride adsorption depends on its concentration in fluid that diffuses to adsorbent’s surface and bonds/held by weak intermolecular forces®. FCDGNC provides good bridge to connect fluoride onto proactive surface of composite that ultimately enhanced sorption capacity. Although, amine of FCDGNC plays major role, however, other functionalities also affect fluoride biosorption due to surface complexation, physic-sorption and chelation that involved affinity to –NH2/OH and made suitable for scavenging fluoride at pH 6.5. Rather, decreased in fluoride sorption in range above pH >6.5 is interpreted as ligand-exchange between fluoride-hydroxide coordinated on immobilized FCDGNC. Thus, FCDGNC displayed a surface controlled monolayer sorption with interactions between fluoride and heterogeneous distribution and diffusion to cationic sites/surface of FCDGNC. The comparative fluoride adsorption capacity of certain chitosan based sorbents and developed FCDGNC is shown in Table 2.
Table 2:Comparative fluoride adsorption capacity of certain chitosan sorbents &FCDGNC.
Adsorption isotherms
Freundlich deals with physico-chemical adsorption on heterogeneous surfaces. Adsorption data were fitted to linearized Freundlich equation is given as: log (x/m)=logKf+1/nlogCe, where x is fluoride adsorbed (mg), m mass of FCDGNC (g), Ce equilibrium fluoride in solution (mg/l) and Kf=constant, measures adsorption capacity and 1/n measures of adsorption intensity of FCDGNC (obtained from slope and intercept of plot between log (x/m) and log Ce). The values of Kf and n were found to be 41.50 and 0.136 for chitosan and 3.27 and 0.67 for FCDGNC with a correlation coefficient (R2) greater than 0.96.
Kinetics of adsorption
Chemical kinetic conditions influenced rate of fluoride adsorption onto chitosan and modeling allowed to estimate sorption rates and guides to suitable rate expressions for characteristic of its possible phenomenon. Thus, pseudo first order, pseudo second order (four linear forms), and the intra-particle diffusion model for kinetics were investigated.
Pseudo first order model
A pseudo-first-order kinetic equation is given as Eqn: where qt is amount of fluoride removed at time t (mg/g), qe is adsorption capacity at equilibrium (mg/g), k1 is pseudo-first-order rate constant (1/min) and t is contact time (min). The plot of ln (qe-qt) versus t. The pseudo-first-order rate constant (k1) determined from the model for the removal of fluoride by FCDGNC at 25 °C.
Pseudo second order model
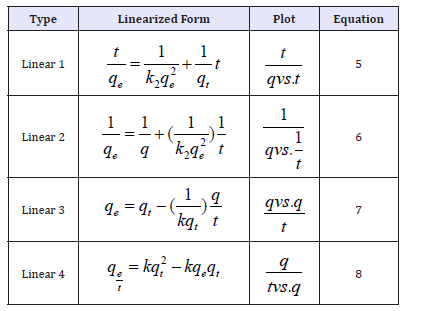
In pseudo-second-order, rate-limiting step is surface adsorption via chemi-sorption, where pollutants get removed due to physicochemical interactions between two phases Sashiwa & Aiba [4]. This model is usually represented by its linear form. Fitted pseudo second order model kinetic parameters for fluoride removal by FCDGNC are calculated by equations 5 to 8, as mentioned in Table 3.
Table 3:Pseudo-second-order kinetic model in linearized forms.
Intraparticle diffusion model
Sorption rate analyzes nature of the ‘rate-controlling step, so intraparticle diffusion model as adsorption dependent on speed of adsorbate diffusion towards FCDGNC and given by Weber and Morris equation: qt=kip t1/2+C where, kip is rate constant of intraparticle transport (g/mg/min) and C is intercept related to thickness of boundary layer (obtained from plot of qt versus t1/2). Comparing kinetics models, pseudo second order model found suitable experimental data and equilibrium reached within 180min, maximum 92% removal with 3.79mg/g fluoride removal capacity with extremely slow diffusion from surface into pores which were least accessible sites for adsorption 66. Results presented in table clearly shows that coefficient of determination for pseudo second order (R2=0.95) is higher than pseudo first order (R2=0.93) and intraparticle diffusion model (R2=0.91). From the plot of intra particle diffusion, the straight lines did not pass through origin suggested unsuitable rate-controlling step. Similarly high k2 obtained by pseudo second order suggested rapid sequestered by FCDGNC functionalities resulted quick equilibrium. The FCDGNC and fluoride involved in rate determining step suggested its chemical adsorption/chemisorptions.
Consequent adsorption data indicated great potential of FCDGNC for de-fluoridation of water. Graphite (20% w/w) doped chitosan yields FCDGNC found better for intrinsic exchange onto – OH/NH2 linkages than that of mere chitosan/graphite adsorbents. De fluoridation capacity is pH dependent and optimum at acidic pH=6.5 than alkaline. Co-anionic impurities like chloride, sulfate, carbonate and bicarbonate adverted defluoridation by competence with fluoride. Equilibrium adsorption data fitted reasonably well for Freundlich model and sorption dynamic followed pseudo-secondorder kinetics indicated complexity i.e., both boundary of liquid film (surface sorption), physic-sorption and intra-particle diffusion contributed to rate-determining step. No graphite leaching found from FCDGNC, rather biocomposite gets regenerated by means of 3g/L of sodium hydroxide in 24hrs agitations which is used for five cycles. Extended sorption properties of FCDGNC pertained with sorption capacity and amount of graphite doped, finds its utility for column defluoridations.
Conclusion
This, develop highly effective and simple to use bio-composite abated fluoride from water and achieve its stringent limit of 1-1.5ppm without affecting treated water quality. Analytical characterization data of FCDGNC showed teh fabrication of -NH2/- OH groups of chitosan with graphite’s laminar layers to improve its compressive strength, thermal stability and obvious sorption onto FCDGNC. This adsorbent showed decreased fluoride biosorption with the increasing of pH >7.5 of medium and sorption fitted well with pseudo-second-order kinetics. Eventually, developed FCDGNC is biodegradable, non toxic, efficient, cheap and benign would be used for water delfuoridation on field/column scale, besides for other anionic mitigation.
Acknowledgment
The author is very grateful to the Head, Department of Chemistry, R.T.M. Nagpur University, Nagpur, M.S., India for providing necessary facilities to carry out this research work.
References
- Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160: 3-14.
- Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27(2): 195-226.
- Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of Hazardous Materials 97(1-3): 219-243.
- Sashiwa H, Aiba SI (2004) Chemically modified chitin and chitosan as biomaterials. Progress in Polymer Science 29(9): 887-908.
- Barber PS, Kelley SP, Griggs CS, Wallace S, Rogers RD (2014) Surface modification of ionic liquid spun chitin fibers for extraction of uranium from seawater: seeking strength of chitin & chemical functionality of chitosan. Green Chem 16(4): 1828-1836.
- Liu B, Wang D, Li H, Xu Y, Zhang L (2011) As (III) removal from aqueous solution using α-Fe2O3 impregnat chitosan bead with As(III) imprinted ions. Desalination 272(1-3): 286-292.
- Zhu HY, Fu YQ, Jiang R, Yao J, Zeng GM, et al. (2012) Novel magnetic chitosan/poly (vinyl alcohol) hydrogel beads: preparation, characterization and application for adsorption of dye from aqueous solution. Bioresource Technolgy 105: 24-30.
- Liu C, Naismith N, Economy J (2004) Advanced mesoporous organosilica material containing microporous β-cyclodextrins for the removal of humic acid from water. Journal of Chromatography A 1036(2): 113-118.
- Reddy DH, Lee SM (2013) Application of magnetic chitosan composites for the removal of toxic metal dyes from solution. Adv Colloid Interface Sci 201-202: 68-93.
- Cui H, Chen J, Yang H, Wang W, Liu Y, et al. (2013) Preparation and application of aliquat 336 functionalized chitosan adsorbent for the removal of Pb(II). Chemical Engineering Journal 232: 372-379.
- Witoon, T, Chareonpanich, M, Limtrakul J (2009) Effect of acidity on the formation of silica-chitosan hybrid materials and thermal conductive property. Journal of Sol-Gel Science and Technology 51(2): 146-152.
- Gupta VK, Suhas A (2009) Application of low-cost adsorbents for dye removal a review. Journal of Environmental Management 90(8): 2313- 2342.
- World Health Organization (WHO) (1984) Fluoride and fluorides: environmental health criteria 36: Geneva, Switzerland.
- Viswanathan G, Gopalakrishnan S, Ilango SS (2010) Assessment of water contribution on total fluoride intake of various age groups of people in fluoride endemic and non-endemic areas of Dindigul District, Tamil Nadu, India. Water Res 44(20): 6186-6200.
- Turner BD, Binning P, Stipp SLS (2005) Fluoride removal by calcite: evidence for fluorite precipitation and surface adsorption. Environ Sci Technol 39(24): 9561-9568.
- Rodriguez DJL, Vladimir A, Rangel M, Shirai K (2009) Synthesis of chitin bio composite for water treatment: Optimization for fluoride removal. Journal of Fluorine Chemistry 130(8): 718-726.
- Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Advance Environmental Research 8(3-4): 553-597.
- Qu J H (2008) Research progress of novel adsorption processes in water purification: a review. Journal of Environmental Sciences 20(1): 1-13.
- Crini G (2005) Recent developments in polysaccharide base materials used as adsorbents in wastewater treatment. Prog Polym Sci 30: 38-70.
- Pandi K, Natrayasamy V (2015) Remediation of fluoride using montmorillonite@chitosan biocomposite. Journal of Chitin and Chitosan Science 3(1): 39-45.
- Swain SK, Dey RK, Islam M, Patel RK, Jha U, et al. (2009) Removal of fluoride from aqueous solution using aluminum-impregnated chitosan biopolymer. Sep Sci Technol 44: 2096.
- Viswanathan N, Sundaram SC, Meenakshi S (2009) Removal of fluoride from aqueous solution using protonated chitosan beads. J Hazard Materials 161(1): 423-430.
- Knaebel KS, Hill FB (1985) Pressure swing adsorption: development of an equilibrium theory for gas separations. Chemical Engineering Science 40 (12): 2351-2360.
- Li Y, Zhang P, Du Q, Peng X, Zhang W, et al. (2011) Adsorption of fluoride from aqueous solution by graphene. Journal of Colloid and Interface Science 363(1): 348-354.
- El Hefian EA, Elgannoudi ES, Mainal A, Yahaya AH (2010) Characterization of chitosan in acetic acid: rheological and thermal studies. Turk J Chem 34: 47- 56.
- Habuda Stanić M, Ravančić ME, Flanagan A (2014) A review on adsorption of fluoride from aqueous solution. Materials 7(9):6317- 6366.
- Alagumuthu G, Veeraputhiran V, Venkataraman R (2010) Adsorption isotherms on fluoride removal: Batch techniques. Arch Appl Sci Res 2(4): 170-185.
- Liang P, Zhang Y, Wang D, Xu Y, Luo L (2013) Preparation of mixed rare earths modified chitosan for fluoride adsorption. Journal of Rare Earths 31(8): 817-822.
- Ngah W, Fatinathan S (2008) Adsorption of Cu(II) in aqueous solution using chitosan bead, chitosan-GLA & chitosan-alginate beads. Chem Eng J 143: 62-72.
© 2018 Rajendra S Dongre. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






