- Submissions

Full Text
Research & Development in Material Science
Improving Bioavailability and Minimizing Ulcerogenic Activity of Dexibuprofen by Using Pectin and Rutin
Zien El-Deen EE1*, Yassin HA 2 and Bahlol M3
1Department of Pharmacy Technology, Tanta University, Egypt
2 Departmentof Pharmaceutics, Egyptian Russian University, Egypt
3 Pharmaceutical Management, Egyptian Russian University, Egypt
*Corresponding author: Zien El-Deen EE, Department of Pharmacy Technology, Tanta University, Tanta, Egypt
Submission: May 14, 2018; Published: July 10, 2018

ISSN: 2576-8840 Volume7 Issue1
Abstract
Dexibuprofen is practically water-insoluble (non-steroidal anti-inflammatory) drug which has a better anti-inflammatory effect than ibuprofen. The aim of the present work was to apply a coating mixture of pectin and rutin to dexibuprofen by utilizing air suspension technique for decreasing its ulcerogenic activity and improving its bioavailability in rats. The bioavailability of the uncoated and coated drug as well as the effect of the coat on the histopathological features of gastric mucosa was determined using male albino rats. Uncoated dexibuprofen administered to rats, histopathological examination revealed that all the animals showed pronounced necrotic gastric mucosa with severe dilated congested blood vessels. However, microencapsulation of dexibuprofen with pectin and rutin mixture had increased the dissolution rate of the drug by about 38% in acidic and 82% in alkaline pH compared to the uncoated drug. Coating the drug with pectin-rutin mixture significantly reduced ulcer incidence as well as ulcer index (p< 0.05) as compared with a free form of the drug in animals administered the same doses. Encapsulating the drug in a carrier with slowing its diffusion into the mucosal media could alleviate the problem of gastric ulceration. Dexibuprofen coated with pectin-rutin mixture administered to rats, histopathological examination revealed that stomach tissue of animals showed normal gastric mucosa. Therefore, Pectin and rutin with dexibuprofen shows higher dissolution as well as higher bioavailability than the free form. Beside that the encapsulated dexibuprofen were found to have lower ulcergenoic activity than the free form of the drug.
Keywords: Microencapsulation; Rutin and pectin; Non-steroidal anti-inflammatory drugs; Fluidized bed technique; Dexibuprofen
Introduction
Dexibuprofen ((S)-(+)-ibuprofen), which has better antiinflammatory effects than ibuprofen and less gastric damage belongs to class II of the Biopharmaceutical classification system (BCS) having low water solubility which is the rate limiting step in absorption of drugs [1,2]. In a series of reports by the authors, the bioavailability, as well as gastric ulcerogenic activity, in rats of some NSAIDs has been evaluated after coating the drug particles with certain types of polymers: cationic and anionic Eudragit [3] as well as cellulose derivatives with acidic, basic and neutral groups [4].
Pectin, edible plant polysaccharides have been shown to be useful for the construction of drug delivery system for specific drug delivery. They are naturally occurring biodegradable polysaccharide consisting of linear chain of 1-4 linked α-D-galactouronic acid residues with varying degrees of methyl ester substitutes [5]. Rutin is used in treating of diseases characterized by capillary bleeding and associated with capillary fragility. It inhibits paw edema induced by egg yolk and its hydroxylation products have been found to inhibit edema and granuloma induced by a variety of irritants [6]. Pectin (polysaccharide) and rutin (glycoside) have been used in treatment of gastric and duodenal ulcers as well as for reduction of permeability and friability of capillaries respectively [7]. The anti-ulcer formulation plantaglucide owes its beneficial effect to its content of pectin [8]. Pectin acts with rutin to form hydrophobic barrier on gastric mucosal membranes [9]. Polysaccharide constitute the majority of the natural-based hydrogel, because of their exceptional properties i.e. biocompatibility, biodegradability, renewability and nontoxicity [10].
A well designed controlled drug delivery system can overcome some of the problems of conventional therapy and enhance the therapeutic efficacy of a given drug. To obtain maximum therapeutic efficacy, it becomes necessary to deliver the agent to the target tissue in the optimal amount in the right period of time thereby causing little toxicity and minimal side effects. There are various approaches in delivery of a therapeutic substance to the target site in a controlled release fashion [11,12]. One such approach is using microcapsules as carriers and drug delivery system for drugs. Microencapsulation is a process by which tiny particles or droplets are surrounded by a coating to give small capsules. In a relatively simplistic form, a microcapsule is a small sphere with a uniform wall around it. The material inside the microcapsule is referred to as the core, internal phase, or fill, whereas the wall is sometimes called a shell coating or membrane. Most microcapsules have diameters ranging from less than one micron to several hundred microns in size [13]. The aim of the present work was to apply a coating mixture of pectin and rutin to dexibuprofen (a NSAID) by utilizing the fluidized bed technique [14]. The bioavailability and ulcerogenic activity of the coated drug particles were evaluated in albino rats and compared with those of the uncoated ones.
Materials
Dexibuprofen (Sigma-Aldrich, St. Louis, Mo, USA) was a gift sample kindly supplied by amriya pharmaceuticals industries, Alexandria, Egypt. The grades of pectin and rutin were purchased from Memphis Co. Egypt. The grade of pectin used was that containing 9 percent methoxy group and 57 percent galacturonic acid, and having a molecular weight of 120.000. Rutin is obtained from the leaves of buckwheat. All other reagents and chemicals were analytical grades and were used as received.
Equipment
Fluidized bed spray granulator equipped with an atomizing nozzle and a special set for coating small particles: Glatt AG, CHprattelen, Switzerland and spectrophotometer: Beckman Mod 24, Beckman Inset. Fullerton, USA. Gas chromatograph: Erba Mom. 2400V, Italy.
Methods
Coating of dexibuprofen with pectin and rutin
Preparation of the coating solution: Seventy-five milliliters of 33.30 percent hydroalcoholic solution containing pectin and rutin, I per cent each, was used for coating dexibuprofen particles [15].
Coating technology: Reviewing the literature about air suspension technique revealed that microencapsulation by this technique reduces processing time and improves the product properties. It was also proven to be more convenient method especially in case of thermo-labile materials. The process consists simply of supporting 30gm drug in the vertical container fluidized from below by a stream of air. The exhaust filter was shaken from time to time to keep the entire drug inside the container. After adjusting the atomized compressed air, the solution of 5% w/v of both pectin and rutin in acetone-isopropyl alcohol mixture (1:1) was sprayed over the bed. The spraying pump was adjusted to be 10rpm to give a suitable droplet size from the sprayed solution. The temperature was maintained at 35-40 °C during the coating process.
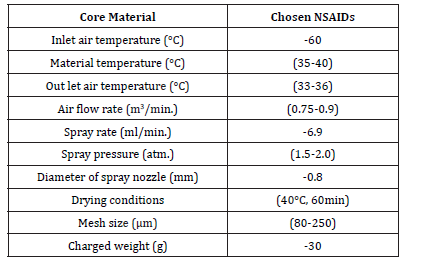
The volume of the solution needed to produce the desirable microcapsules was 200ml. When the microcapsules have been formed, the spray was turned off and the product was left to fluidize inside the apparatus for about 60 minutes for complete drying at the same temperature. The encapsulated particles were stored in a desiccator over anhydrous calcium chloride for 48hrs before any further study. Table 1 shows the operating conditions in coating dexibuprofen as a model NSAID.
Table 1:Operating conditions in coating the chosen NSAIDs.
In-vitro Study
Screening of the microcapsules
The particle size of microcapsules was determined by sieving a known weight of the obtained microcapsules on a mechanical shaker with a nest of standard sieves ranging in size from 0.063 to 1.6mm.
In-vitro drug release studies
The dissolution rate of dexibuprofen microcapsules was studied using USP dissolution test apparatus employing paddle type (Paddle type, Copley, England). Accurately weighed samples of microcapsules were used which were calculated to contain 50mg of the tested drug. Microcapsules were placed in 900ml of dissolution media (two types’ 0.1N HCL (pH 1.0) and phosphate buffer (pH 7.4)). Paddle speed of 100rpm and temperature of 37.5ᵒC±0.2 were employed. Aliquots (5ml) were withdrawn from the dissolution medium, filtered through 0.45μm membrane filter and replaced with equal volumes of pre warmed fresh media to maintain constant volume and keep sink condition [16]. The drug concentration and the percentage drug released were determined with respect to time spectrophotometrically at 222nm [17]. The in-vitro dissolution studies were performed in triplicate for each sample and the results were reported as a mean ±SD.
Bioavailability
Uncoated as well as coated drug were administered to two groups each of 28 male albino rats weighing 250±10g. The animals were fasted for 20h prior to the experiment but had free access to water. Each treatment was suspended in 1 percent w/v methylcellulose solution at a concentration of 25mg/ml. A dose level of 50mg/kg was kept constant for all the treatments. Control animals were dosed with an equivalent volume of 1 per cent methylcellulose solution free from drug. Four animals were scarified at time intervals of 1, 2, 3, 5, 7, 9 and 12h. Blood (TSU) Institutional animal care and use committee (TSUIACMC Protocol 10-080).
In-vivo Ulcerogenicity Studies
Experimental animals
Male Wistar rats, weighing 180-200gm, were obtained from national researches center (Cairo, Egypt). Rats were maintained at 22±1 ᵒC on a 12h light-dark cycle allowed rat chow and water ad libitum. The allocation of animals to all groups was randomized. In-vivo experimental protocols had the approval of the institutional animal ethics committee (IAEC) (IAEC/PROPOSAL/DB-4/2010).
Before the start of the experiments, rats were housed individually in wire mesh cages to avoid coprophagy under controlled environmental conditions. Food was withdrawn for 36h but water was allowed ad libitum [18,19]. The absence of ulcers in some of the treated groups has revealed that the pre-fasting conditions alone doesn’t induce ulcer.
In case of long term drug administration, the same procedure was followed except that the animals were kept on normal diet along the time of 30 days while administering a daily dose level of 1.06mg/ kg [20-22]. The animals subjected to long term administration were kept under constant observation throughout the experiment. The bioavailability was calculated as previously reported [18]. At the end of the experiment, the abdomen was opened. Each stomach was excised, dissected along the greater curvature and contents were emptied by gently rinsing with isotonic saline solution. Each stomach was pinned out on a flat surface with the mucosal surface uppermost [23].
Macroscopic examination of gastric ulcers
The ulcer incidence, represented as hemorrhagic lesions and gastric ulcers were examined and assessed macroscopically with the help of a 10x binocular magnifier immediately after the animals were sacrificed. To quantify the induced ulcers in each stomach, the scoring systems described in the literature [6,24] were employed. The induced ulcers in these experiments were in the form of small spots punctiform lesions and thus each was given a score between 1mm and 4mm. Ulcers of 0.5mm diameter were given a score of 1mm whereas ulcers of diameters 1mm and 2mm were given scores of 2mm and 4mm, respectively. Stomach with no pathology was assigned a score of zero. For each stomach, an ulcer index was calculated as the sum of the total scores of ulcers. Six determinations were made for each drug suspension administered. The average ulcer index is presented as the mean (n=6)±SD.
Histopathological examination of stomach sections
For histopathological examination, the stomach was surgically extirpated from each group and opened through vertical incision along the greater curvature and photographs were taken of the inside surface of the stomach. The stomach tissues were then washed in 0.9% saline and a portion of it was kept in 10% buffered formalin solution for histopathological studies. The sections were then stained with hematoxylin and eosin. The tissue sections were examined under an Olympus BX51 (Olympus Corporation, Tokyo, Japan) microscope and images were captured with a digital camera attached to the microbeads [14].
Statistical Analysis
One way ANOVA test followed by Tukey posttest was used for comparisons between the treatment and control groups. Data were presented as Mean±SD. The P values < 0.05 was considered as significance level during this study. Previous work in our laboratory showed that there is no chemical interaction between the adopted drugs and polymers utilizing infrared analysis (IR) and differential scanning calorimetry (DSC) techniques. The in-vitro release of drugs from different microbeads was also followed in our previous work [17].
Results and Discussion
Size distribution of microcapsules
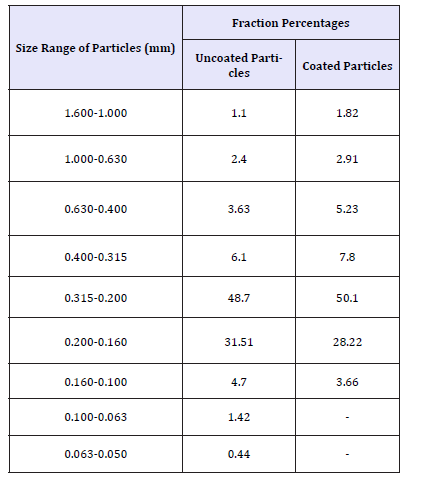
Table 2 shows the particle size distribution of uncoated and coated dexibuprofen.
Table 2:Particle size distribution of uncoated and coated dexibuprofen.
In- vitro release results
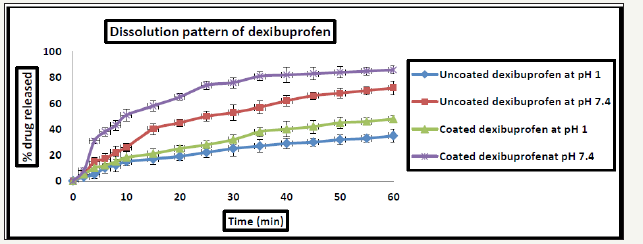
Microencapsulation of dexibuprofen with pectin and rutin mixture had increased the dissolution rate of the drug by about 38% in acidic and 82% in alkaline pH respectively compared to the uncoated drug. The release profile of dexibuprofen from the proposed formulations is shown in Figure 1.
Figure 1: Dissolution pattern of both uncoated and coated dexibuprofen at different pH values *All data are Mean±SD, n=3.
Bioavailability results
The plasma levels at different time intervals for rats administered coated and uncoated drug are illustrated in Figure 2. From Figure 2, drug concentration in rat serum was found to be nearly doubled after coating the drug with pectin-rutin mixture. Table 3 shows the peak height, peak times as well as AUC0-12 of both coated and uncoated drug.
Figure 2: Serum level of both uncoated and coated dexibuprofen. *All data are Mean±SD, n=3.

Table 3:Bioavailability criteria of uncoated and coated drug after systemic administration to rats

*The results are average of four readings.
In-vivo results
Macroscopic observation: Macroscopic examination of rat stomachs of the control group, uncoated as well as coated drug are presented in Figure 3. From Figure 3 it is obvious that, gross study of gastric lumina of the control group showed completely an apparent normal gastric mucosa regarding a normal rauga and mucous covering layer (Figure 3a). A rat stomach which was administered a dose of (50mg/kg) of dexibuprofen, showed pin point hemorrhagic area as well as a wide spread hemorrhaging as indicated by the red spots which are blood clots (Figure 3b). A rat stomach which was administered dexibuprofen coated with pectinrutin mixture showed a normal gastric mucosa with a small area of congestion (Figure 3c).
Figure 3: Representative images showing morphological changes in rat gastric tissues after administration of the drug.
3a: control.
3b: uncoated.
3c: coated dexibuprofen.

Effect of both coated and uncoated dexibuprofen on ulcer incidence as well as ulcer index in rats: From the Table 4, it is evident that, coating of the drug with pectin-rutin mixture significantly reduced ulcer incidence as well as ulcer index (p< 0.05) as compared with a free form of the drug in animals administered the same doses. The obtained results indicate that, encapsulating the drug in a carrier as well as slow diffusion of the drug into the mucosal media could alleviate the problem of gastric ulceration. From the Table 4, it is evident that, coating of the drug with pectin-rutin mixture significantly reduced ulcer incidence as well as ulcer index (p< 0.05) as compared with a free form of the drug in animals administered the same doses. The obtained results indicate that, encapsulating the drug in a carrier as well as slow diffusion of the drug into the mucosal media could alleviate the problem of gastric ulceration.
Figure 4: Representative images showing histological observations in rat gastric tissues after administration of the drug
4a: Control
4b: Uncoated
4c: Coated dexibuprofen.
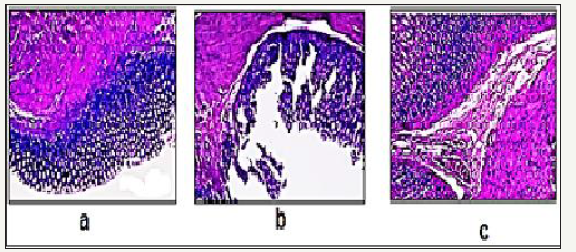
Histolopathological examination: The histopathological pattern of the mucosal specimens was studied by examining the histology of the treated and control samples. Effect of both uncoated as well as coated dexibuprofen on stomach tissue histopathology is presented in Figure 4. Histopathological examination of Hx & E stained stomach sections of control rats (n=6), revealed that all the six animals showed completely normal gastric mucosa with excess mucous layer (Figure 4a). In uncoated dexibuprofen administered rats (n=6), histopathological examination revealed that all the six animals showed pronounced necrotic gastric mucosa with sever dilated congested blood vessels in the lamina properia with severe edema infiltrated by inflammatory cells (neutrophil infiltration), also superficial mucosal layer showed marked congestion, necrosis (Figure 4b). In case of dexibuprofen coated with pectin-rutin mixture administered rats (n=6), histopathological examination revealed that stomach tissue of animals showed near normal gastric mucosa (Figure 4c).
Discussion
Drug released from the obtained pectin-rutin gel beads was affected not only by the esterification degree of pectin but also by the blend composition [6]. The increase of drug release from the coated particles compared with the uncoated particles could be attributed to a lax structure of pectin-rutin hydrogel network. Drug release from beads made of pectin is substationally faster, due to a lower and weaker cross-linking, mainly by hydrogen bridges [25].
Ali et al. [26] showed that in calcium and zinc pectinate beads rutin release was dependent on the proposed formulation as well as pH of the medium. Pourjavadi et al. [9] proved that pectin forms a certain type of complex with either rutin or its aglycone quercetin. Pectin-quercetin (Quertin) was proved to have anti-ulcerogenic activity [6]. Incorporation of dexibuprofen with pectin and quercetin in the form of formulated tablets led to increase in the dissolution of the drug in-vitro. The bioavailability has been greatly increased and the ulcerogenic activity decreased in rabbits administered the formulation [9]. These effects were assumed to be due to the formation of a complex (Butaquertin) between pectin, quercetin and dexibuprofen. The increased dissolution and bioavailability were attributed to be due to certain surface-active properties of the complex acting like micelles. Later, similar explanations were given to the effect B-cyclodextrin on the bioavailability of sulindac done by Yusuf et al. [27]. The influence of pectin and rutin mixture on the dissolution and bioavailability of dexibuprofen could be described on the same basis, although a different technique was involved.
Conclusion
The purpose of the present work was to develop drug delivery system containing pectin and rutin for improving the bioavailability and minimizing the ulcerogenic activity of the insoluble dexibuprofen utilizing the air suspension technique. From the results of characterization and drug release studies it is concluded that pectin-rutin matrix enhances the release of dexibuprofen at the different pH values. The proposed combination of the drug with pectin and rutin showed a pronounced effect on the bioavailability of the drug in experimental animals as well as a pronounced decrease of the ulcerogenic activity of the drug.
References
- Adams SS, Bresloff P, Mason CG (1976) Pharmacological differences between the optical isomers of ibuprofen evidence for metabolic inversion of the (-) isomer. J Pharm Pharmacol 28(3): 256-257.
- Bomabello A, Galmozzi MR, Canaparo R, Isaia GC, Serepe L, et al. (2003) Dixibuprofen (s+-isomer ibuprofen) reduces gastric damage and improves analgesic anti-inflammatory effects in rodents. Anesth Analg 97(2): 402-408.
- Zien E, Yassin H (2017) Role of different types of polyacrylic polymers on decreasing the ulcerogenic effect of certain non-steroidal antiinflammatory drugs. JPRI 20(4): 1-10.
- Zien ED, Ghorab M, Gad SH, Yassin H (2015) Effect of different polymers on the release of diclofenac sodium controlled release drug delivery system. J Pharm Pharmaceut Sci 3(1): 1-11.
- Costas L, Pera M, Lopez A, Mechetti M, Castro G, et al. (2012) Controlled release from smart pectin gel microspheres under physiological stimulated fluids. Appl Biochem Biotechnol 167(5): 1396-1407.
- Meshali M, Zein E, Omar S, Luzzi L (1992) A new approach to encapsulating non-steroidal anti-inflammatory drugs. V Biopharmaceutical study of microcapsules of azapropazone coated with pectin and rutin. J Microencapsul 9 (1): 67-72.
- Michelle C, Woodward K, Huff, Frank G, Michael L, et al. (2014) Effect of pectin, lecithin, and antacid feed supplements (Egusin®) on gastric ulcer scores, gastric fluid pH and blood gas values in horses. BMC Vet Res 10(Supp 1): S1-S4.
- Saumendu D, Jashabir C, Dibyendu S, Sumit D, Narzima B, et al. (2013) Herbs used in peptic ulcer: a review. Int J of Pharm Res & Allied Sci 2(2): 9-23.
- Pourjavadi A, Barzegar S (2009) Smart pectin-based superabsorbent hydrogel as a matrix for ibuprofen as an oral non-steroidal antiinflammatory drug delivery. Starch 61(3-4): 173-187.
- Mohkam M, Allahveridi M (2004) Controlled release of biomolecules from pH-sensitive network polymers prepared by radiation polymerization. J Drug Target 12(3): 151-156.
- Jyothi S, Seethadevi A, Suria K, Muthuprasanna P (2012) Microencapsulation a review. International Journal of Pharma and Bio Sciences 3(1): 509-531.
- Poshadri A, Aparna K (2010) Microencapsulation technology: a review. J Res Angru 38(1): 86-102.
- Nitika A, Ravinesh M, Chirag G, Manu A (2012) Microencapsulation-a novel approach in drug delivery: a review. Indo Global Journal of Pharmaceutical Sciences 2(1): 1-20.
- Zien E, Rashedy M, Ghorab M, Gad Sh, Yassin H (2015) In vivo evaluation of ulcerogenic activity of ketorolac, its solid dispersion systems, as well as its microcapsules in rats. J Pharm Pharmaceut Sci 4(3): 23-37.
- Wong S, Kellaway I, Murdan S (2006) Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing micro particles. Int J Pharm 17(1): 61-68.
- Raval K, Bagda A, Patel J, Paun J, Chaudhari K (2010) Preparation and evaluation of sustained release ninmesulide microspheres using response surface methodology. Journal of Pharmacy Research 3(3): 581-586.
- Houssieny E, Dien E, Messiry H (2014) Enhancement of solubility of dexibuprofen applying mixed hydrotropic solubilization technique. Drug Discov Ther 8(4): 178-184.
- Prabagar B, Beom L, Hoon O, Jong W, Soon Y, et al. (2009) Enhanced oral bioavailability of dexibuprofen by a novel solid Self-emulsifying drug delivery system (SEDDS). Eur J Pharm Biopharm 72(3): 539-545.
- El-shitany N (2006) Mechanism of omeprazole induced gastric protection against ethanol induced gastric injury in rats; role of mucosal nitric oxide and apoptotic cell death, Proceeding of 1st international Egyptian jordanian conference on biotechnology and sustainable development: current status & future scenarios. Medical & Pharmaceutical 2:183-193.
- Bhargava K, Gupta M, Tangri K (1973) Mechanism of ulcerogenic activity of indomethacin and oxyphenbutazone. Eur J Pharmacol 22(2): 191-195.
- Schmassmann A, Peskar B, Selter C, Netzer P, Stroff T, et al. (1998) Effect of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br J Pharmacol 123(5):795-804.
- Brzozowski T, Konturek P, Konturek S, Sliwowski Z, Pajdo R, et al. (2001) Classic NSAIDs and selective cyclooxygenase (COX-1) and (COX-2) inhibitors in healing of chronic gastric ulcers. Microsc Res Tech 53(5): 343-353.
- Alsarra I, Ahmed M, Alanazi F, Tahir K, Alsheikh A, et al. (2010) Influence of cyclodextrin complexation with nsaids on nsaids/cold stress-induced gastric ulceration in rats. Int J Med Sci 7(4): 232-239.
- Zien El, Ghorab M, Gad S, Yassin H (2015) Effect of certain polymers on the ulcerogenic activity of a non-steroidal anti-inflammatory drug. World J Pharm Res 4(6): 2275-2290.
- Hagesaether E, Bye R, Sande S (2008) Ex vivo mucoadhesion of different zin-pectinate hydrogel beads. Int J Pharm 347(1-2): 9-15.
- Ali A, Odile C, Philippe C (2011) Drug release from calcium and zinc pectinate beads: impact of dissolution medium composition. Carbohydrate polymer 85(2): 388-398.
- Yusuf AH, Sanaa A, Esmat, Nagla A, Mohamad A (2016) Sulindac solid dispersion: development, characterization and in vivo evaluation of ulcerogenic activity in rats. J App Pharm Sci 6(2): 022-031.
© 2018 Zien El-Deen EE . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






