- Submissions

Full Text
Research & Development in Material Science
Graphene-The Wonder Material
AS Khanna*
Department of Metallurgy, IIT Bombay, India
*Corresponding author: AS Khanna, Department of Metallurgy, IIT Bombay, India
Submission: March 26, 2018; Published: May 24, 2018

ISSN: 2576-8840 Volume6 Issue4
Abstract
A very recent article projected the global market for Graphene would be 207.8 billion by 2020. What is so great about Graphene? Where Graphene can be used? Are there economical methods to make industrial scale grapheme? These are the few questions which need to be answered.
What is Grapheme?
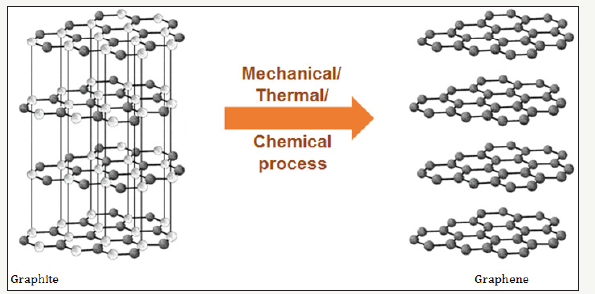
It is a 2 dimensional atomic crystalline layer separated from graphite. It is an allotrope of carbon in the structure of a plane of sp2 bonded atoms with a molecule bond length of 0.142 nanometres. In fact graphite consists of millions of thin graphene layers bonded together by strong Vanderwall forces with interplaner spaces of about 0.335 nanometers. In 2010, two scientists from university of Manchester were able to separate a single layer of graphene using a scotch tape which resulted in Nobel Prize to them. Today one of the most important methods of graphene synthesis is by exfoliation (force separation) of graphene layers. This is shown schematically as (Figure 1).
Figure 1:Schematic of Graphene and its relation to graphite.
What Benefit One Gets from This Separation from Graphite?
Graphite is a conducting material however, the conductivity of graphene increases several fold when it is separated from graphite as a single layer. Graphene is one atom thick, the lightest material known. It is also the strongest material with young’s modulus ~1,100GPa and has high fracture strength of 125GPa. It is the best conductor of heat at room temperature 3000Wm/K and also the best conductor of electricity. Other notable properties of graphene are its unique levels of light absorption at πα≈2.3% of white light, and its potential suitability for use in spin transport.
Because of these unique properties graphene is being considered an important material for several applications in different industries ranging from micro-electronics, heat conduction-room heaters, batteries, graphene membranes which can be used to separate sea water to normal water in a single step. We have found, however, unique new application of graphene in organic coatings.
Two types of coatings have been designed:
Pre-treated graphene layer
A single layer of graphene can be made on metals with very strong adherence, in fact chemical bonding with substrate metal.
This however requires some kind of fictionalization to be carried out on graphene so that it can be attached to a polar metal surface. Successful 2 micron graphene layer was made on steel sample with excellent chemical bonding and long durability. It passed 2000h salt spray test with corrosion rate lower than 1μA. It also has water permeability lower than an order of magnitude from pure silane coating [1].
This coating has tremendous potential applications in automobile sector and for aluminium coil coating. In automobile coatings, it can replace the ED coating while in aluminium coil coating, it can replace the chromate coating (CrVI ).
Graphene Based Primer Coatings
Zinc base primer coatings are well known. Zinc rich epoxy with 72-76% zinc powder in epoxy and Inorganic Zinc Silicate (IOZ) with 82-86% Zinc powder in ethyl silicate resin is too well known primers. These are the primer coatings which are applied on all external steel structures as primer coatings to provide cathodic protection in case the coating is broken and thereby provide long durability. All Zinc based coatings can be replaced with graphene based coating with just 1.5% graphene powder addition in epoxy. Further, it gives a life which is 25 times that of a Zinc rich coating and about 5 times that of an IOZ coating [2]. This coating has been well tested in the laboratory and has also been used on a structure exposed to C5 environment.
Production of graphene
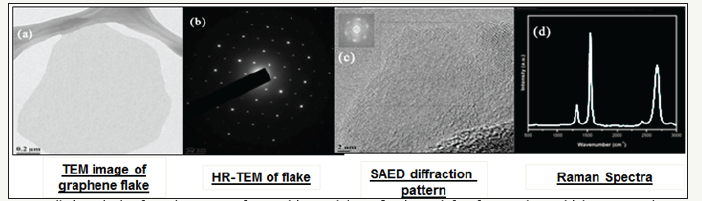
In order to make such coatings, large amounts of graphene powder is required. We therefore synthesised industrial scale graphene using a pressure exfoliation method with a yield of more than 18% (about 10% more than the conventional exfoliation high shear method). The quality of graphene is excellent as shown in the enclosed Figure 2 with TEM picture, electron diffraction and Raman spectroscopy. The graphne formed is defect free and is a three atomic layer thick layer.
Figure 2:Detailed analysis of graphene manufactured in our lab confirming a defect free 3-4 layer thick pure graphene.
Conclusion
In summary it can be said that graphene age is coming. Next decade will exploit graphene for various industrial applications. Simultaneously, efforts will be made to search cost effective methods of graphene manufacturing so that it is easily available for various potential applications.
References
- Aneja KS, Khanna AS (2015) Graphene based Coatings against Cr (VI) replacement. Nanoscale 7(42): 17879-17888.
- Aneja KS, Böhm HLM, Khanna AS, Böhm S (2016) Functionalised graphene as a barrier against corrosion. Flat Chem 1: 11-19.
© 2018 AS Khanna . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






