- Submissions

Full Text
Research & Development in Material Science
Protein Release Systems for Bone Regeneration
Diana Daniel Pérez and Fernando Gomes de Souza Junior*
Institute of Macromolecules, Federal University of Rio de Janeiro, Brazil
*Corresponding author: Fernando Gomes de Souza Junior, Institute of Macromolecules, Professor Eloisa Mano, Federal University of Rio de Janeiro, Technology Center-University City, Av Horacio Macedo-2030, Brazil
Submission: March 07, 2018;Published: May 08, 2018

ISSN: 2576-8840
Volume5 Issue5
Abstract
Therapeutic proteins have emerged with a significant role in the treatment of a broad spectrum of diseases, including metabolic disorders, autoimmune diseases, and bone regeneration. However, the efficacy of therapeutic proteins is limited by their immunogenicity, instability and short half-life. Nowadays, the use of therapies such as bone morphogenetic proteins (BMP) is limited by the inefficient delivery. The high doses applied are frequently related to adverse and severe effects. Aiming to overcome these limitations, the use of polymers as matrixes provide several strategies for the application of therapeutic proteins in organisms. This work presents the main used polymers besides collects some essential aspects related to therapeutic proteins used in bone regeneration.
Introduction
Proteins are widely used for therapeutic purposes [1]. However, clinical applications of the proteins are restricted by their potential immunogenicity that limits their therapeutic efficacy besides threatens patients with adverse effects. Additional limitations of therapeutic proteins are their degradation through circulation, glomerular filtration, and processing by the immune system, thus leading to a low plasma half-life, poor bioavailability and reduced in vivo activities [2-5]. These problems require frequent injection and the use of large doses to achieve the required therapeutic efficacy, increasing the risk of producing severe allergic responses [6-10]. To overcome these limitations, micro and nanoencapsulation technology using polymers as continuous phase provide several strategies for the application of therapeutic proteins in organisms [11,12].
Polymer science has a salubrious impact on the encapsulation of therapeutic proteins since polymers are the primary materials used to prepare these systems [13-17]. Thus, the search for new properties of these materials based on obtaining new derivatives from the commonly used polymers or new blends of them is a goal of many researchers [18-22]. Biopolymer-based nano and microparticles are being more and more considered as essential carriers of biological agents because of their advantages of biocompatibility, ease of surface modification, localized action and reduced systemic toxicity [23,24]. Microencapsulation of proteins in polymer matrices requires special care due to the fragile nature of these molecules. Each protein family has different characteristics, requiring the adaptation of techniques and materials to their satisfactory encapsulation [25-27]. In this work, an overview of the main therapeutic proteins used in bone regeneration will be presented.
Proteins and Bone Regeneration
Proteins are natural polymers present in all living cells [28,29]. They are the most abundant organic molecules in living systems. These molecules are much more diverse in structure and function than other classes of macromolecules. The use of proteins aiming therapeutic purposes has increased. However, the use of proteins as therapeutic agents presents some limitations, being very sensitive to environmental conditions, e.g., their physicochemical instability in some body fluids (in saliva and gastric juices) which limits the use of specific routes of administration, as the oral one. Likewise, the large size of these biomolecules limits their transdermal use. The most common mode of administration of these drugs is through intravenous injections, which are often not well tolerated by the patient. In addition, most of these proteins have short halflives in the bloodstream and need to be frequently given in high doses for efficacy, but such systemic administration in high doses can cause side effects [30,31], such as restlessness, hyperhidrosis, lightheadedness, itching and rash, tachycardia and arrhythmias, and so forth [32-34].
Among the numerous proteins that have already been used as therapeutic agents, those used for bone regeneration are a crucial group. The bone morphogenetic proteins (BMPs) are glycoproteins found in bone tissue. BMPs were first described by Marshall Urist in 1960 when the formation of bone after implantation of the demineralized bone matrix at intramuscular points was noticed. BMPs are responsible for the induction and regeneration of demineralized bone grafts. They are involved in the regulation of the differentiation processes of several cells during skeletal development and fracture as well [35,36]. BMPs are used as a tool for the treatment of degenerative trauma, neoplastic conditions, mandibular reconstruction, oral and maxillofacial surgeries [37].
The Food and Drug Administration (FDA) approved the use of BMPs in July 2002 for the treatment of traumatic, neoplastic, and infectious degenerative diseases. Its application had a rapid increase in the United States despite the high initial cost due to the excellent obtained results [38].
Main bone morphogenetic proteins
Figure 1:Main bone morphogenetic proteins and respective characteristics.
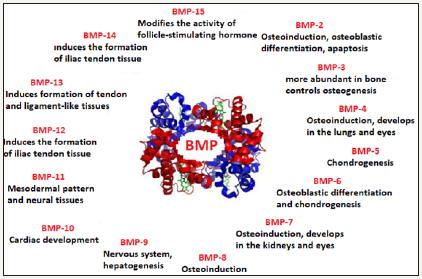
There are several types of BMP with the function of producing bone. Figure 1 shows the main bone morphogenetic proteins and respective characteristics. The types of BMP-2 to BMP-9 belong to the super family transforming growth factor beta. Sequential activation of all BMPs is vital for osteoinduction and bone callus formation. In this specific context, BMP-2, BMP-4, and BMP-7 present the highest chondroinductive and osteoinductive activities. Many processes for cell growth (apoptosis and differentiation) depend on the BMP signaling. These proteins play essential roles in maintaining adult tissue, such as fracture repair initiation, and vascular remodeling [39].
In turn, among BMPs, BMP-2 and BMP-9 are the most active proteins for inducing the activation of alkaline phosphatase, being fundamental for the differentiation of enzymes and progenitor cells into osteoblasts. Besides that, BMP-2 and BMP-9, present potent osteoinductive activity. In turn, BMP-2 plays a crucial role in the processes of chondrogenesis and osteogenesis, as well as revascularization and is considered essential in the repair of fractures. On the other hand, BMP-3 does not induce the formation of specific tissues, being useful to the cartilage formation [40,41].
BMP systems also play a crucial role in folliculogenesis. Specifically, BMP7 is highly expressed in the cell layer of the ovarian follicles. This protein also acts as an endogenous suppressor of new glioblastoma cells (GPC) and thus can become a new cancer treatment [42]. The growth factors BMP4 and BMP7 affect the function of granulosa cells directly. On the other hand, they suppress the apoptosis of these cells [43].
Currently, there are several studies in the literature proving the use of human recombinant morphogenetic protein-2 (rhBMP-2) with good bone neoformation results, especially in critical defects and in sites with difficult conventional bone grafting [44-55]. Concretely, in the area of dentistry, rhBMP-2 is used to enhance regenerative results in surgeries of significant bone defects in the mandible or maxilla, periodontal surgery, surgical resection associated with tumor lesions and adaptation of dental implants [56].
Protein Release Systems
As already mentioned, the effectiveness of the proteins will depend on their bioavailability at the desired binding site, which depends on the structural and biomechanical properties of the carriers. As those proteins are relatively soluble, if a suitable carrier cannot keep them trapped, they will be eliminated before reaching the desired site. Also, this premature release can cause side effects such as ectopic bone growth, inflammation, and uncontrolled bone formation [57].
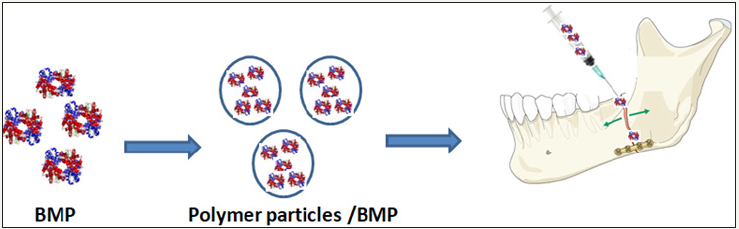
Therefore, more in-depth research related to the use of polymer particles as transport systems for these species should be increasingly pursued. In addition to the different techniques of encapsulation since it will offer new functional properties to this asset, as it is controlled release in a specific environment, keeping the asset isolated, increasing its useful life [58]. Figure 2 shows the use of nanoparticles containing BMP for fracture treatments.
Microencapsulation techniques
Microencapsulation is the name of a set of techniques for preserving the quality of delicate substances through a method able to produce materials with remarkable new properties. Aiming this, usually, a polymer shell is prepared on the active substance using a specific technique [59]. A bibliometric research using Google Scholar, performed in March 2018, allowed inferring that from more than 203k documents related to master key “Protein release and microparticles”, 24.7%, 18.0% and 7.4% of them used the emulsion, spray drying, and coacervation techniques for the preparation of the microparticles. Therefore, these three techniques are responsible for 50.1% of the studies related to the microencapsulation of proteins. Further details about the microencapsulation techniques were already presented in another work of our group [60].
Figure 2: Use of polymer particles containing BMP for fracture treatments.
The release of proteins from polymer particles
Pursuing to preserve the quality of delicate substances, several researchers carried out a myriad of works where they used these different microencapsulation techniques aiming to prepare systems for the controlled release of a protein using polymers as the protective shell [61-68]. The main polymers used for the release of proteins are poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA). More specifically, a second bibliometric research using Google Scholar, also performed in March 2018, allowed inferring that from more than 987k documents related to master key “bone tissue regeneration proteins”, 15.4% of them used polymers as the matrix of the delivery system. So, more than 152k documents described the use of several polymers for this purpose. From them, PLA corresponds to 32.1% of the total. In turn, PLGA, polyurethane, and poly(methyl methacrylate) correspond to 23.1%, 13.9%, and 9.1%, respectively. Therefore, these four polymers are responsible for 78.2% of the studies. Some representative examples using polymer materials and microrencapsulation techniques used for the BMPs release are here presented:
Quinlan et al. [69] used the Spray drying technique to encapsulate the BMP-2 protein in polymer matrices. The objective of the work was to evaluate the potential of alginate and PLGA as BMP-2 transporters. They used the double emulsion technique for PLGA particles. PLGA ensured the stabilization of the protein and allowed its controlled release after implantation into a collagenhydroxyapatite (CHA) framework. The obtained microparticles presented diameter between 1 and 10μm. The morphology of the materials is a function of the preparation procedure used. The alginate microparticles presented an irregular shape with a smooth surface, while the PLGA microparticles had a spherical and porous shape. After the release study, the author inferred that alginate and PLGA microspheres released 46% and 12% of their total contents, respectively. Besides, after incorporation of these microparticles into the frameworks, the BMP-2 protein was sustained release for up to 28 days.
Qiao et al. [70] used poly (lactic-co-glycolic acid) (PLGA) to create a controlled release system of the BMP-2 protein by the double emulsion-solvent evaporation (W/O/W). The authors encapsulated the protein with the calcium phosphate cDNA (CaPi) plasmid complex that was used to promote transfection of the protein. Dynamic light scattering (DLS) showed that the particle size of calcium phosphate was about 350nm and the particle size of BMP-2 / CaPi was about 600nm. The encapsulation efficiency ranged from 30 to 50%. The initial release rate in 24 hours was less than 7.5%. PLGA-BMP2/CaPi microspheres released the BMP-2 cDNA plasmid for up to 30 days. The authors suggested that PLGABMP2/ CaPi microspheres may promote ectopic osteogenesis, with strong prospects for the promotion of bone regeneration.
Lochmann et al. [71] used block copolymers of polyethylene glycol - poly (lactic-co-glycolic acid) (PEG-PLGA) to produce rhBMP-2 protein controlled release systems. They were prepared by the double emulsion-solvent evaporation technique. The authors investigated the influence of a co-solvent, PEG 300, on the properties of the particles. The results were compared to data from unmodified PLGA microspheres. In this study, the authors were able to encapsulate 85% rhBMP-2 in PLGA microspheres. Thus, the authors concluded that PEG-PLGA microspheres have promising application in sustained release allowing reduction of the required dose of rhBMP-2 to limit adverse effects and costs. Also, the data indicated that the use of PEG as the internal phase co-solvent is not suitable for rhBMP-2.
Conclusion
The use of Bone Morphogenetic Proteins has expanded the treatment options to achieve the regeneration of bone tissue, producing a tremendous impact in human bone reconstruction. Several works about BMPs using release systems based on different polymers as a means of transport presented positive results demonstrating the osteoinductive capacity of the proteins. Most of them were prepared using the combination of PLA and microencapsulation by emulsion technique. Therefore, this combination must be intensely studied aiming to produce innovative materials, which will improve the human quality of life.
Acknowledgment
The authors thank to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Financiadora de Estudos e Projetos (FINEP PRESAL Ref.1889/10) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for the financial support and scholarships.
References
- Kardani K, Bolhassani A (2018) HPV Proteins and Their Functions. HPV Infect Diagn Prev Treat 8.
- Kopp MR, Arosio P (2018) Microfluidic approaches for the characterization of therapeutic proteins. J Pharm Sci 107(5): 1228-1236.
- Moreno AM, Palmer N, Aleman F, Chen G, Pla A, et al. (2018) Exploring protein orthogonality in immune space: a case study with AAV and Cas9 orthologs. Bio Rxiv 245985.
- Tran K, Gurramkonda C, Cooper MA, Pilli M, Taris JE, et al. (2018) Cellfree production of a therapeutic protein: Expression, purification, and characterization of recombinant streptokinase using a CHO lysate. Biotechnol Bioeng 115(1): 92-102.
- Felgner S, Kocijancic D, Frahm M, Heise U, Rohde M, et al. (2018) Engineered salmonella enterica serovar typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Onco Immunology 7(2): e1382791.
- Santos AF, Du Toit G, Douiri A, Radulovic S, Stephens A, et al. (2015) Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut. J Allergy Clin Immunol 135(1): 179-186.
- Vandenplas Y, Brueton M, Dupont C, Hill D, Isolauri E, et al. (2007) Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch Dis Child 92: 902-908.
- Sala-Cunill A, Björkqvist J, Senter R, Guilarte M, Cardona V, et al. (2015) Plasma contact system activation drives anaphylaxis in severe mast cellmediated allergic reactions. J Allergy Clin Immunol 135(4): 1031-1043.
- Kleine-Tebbe J, Wangorsch A, Vogel L, Crowell DN, Haustein UF, et al. (2002) Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1-related PR-10 protein in soybean SAM22. J Allergy Clin Immunol 110(5): 797-804.
- Cordle CT (2004) Soy protein allergy: incidence and relative severity. J Nutr 134(5): 1213S-1219S.
- He H, Ye J, Wang Y, Liu Q, Chung HS, et al. (2014) Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. J Controlled Release 176: 123-132.
- Zhang P, Sun F, Tsao C, Liu S, Jain P, et al. (2015) Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc Natl Acad Sci USA 112: 12046-12051.
- Kumar MNR (2000) A review of chitin and chitosan applications. React Funct Polym 46(1): 1-27.
- O’hagan DT, Rahman D, McGee JP, Jeffery H, Davies MC, et al. (1991) Biodegradable microparticles as controlled release antigen delivery systems. Immunology 73(2): 239-242.
- Liu X, Sun Q, Wang H, Zhang L, Wang JY (2005) Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials 26(1): 109-115.
- Yang YY, Chung TS, Ng NP (2001) Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/ evaporation method. Biomaterials 22(3): 231-241.
- O’Hagan DT, Jeffery H, Davis SS (1994) The preparation and characterization ofpoly (lactide-co-glycolide) microparticles: III. Microparticle/polymer degradation rates and the in vitro release of a model protein. Int J Pharm 103(1): 37-45.
- Bexiga NM, Bloise AC, Alencar AM, Stephano MA (2018) Freeze-drying of ovalbumin-loaded carboxymethyl chitosan nanocapsules: Impact of freezing and annealing procedures on physicochemical properties of the formulation during dried storage. Dry Technol 36(4): 400-417.
- Lee C, Choi JE, Park GY, Lee T, Kim J, et al. (2018) Size-tunable proteinpolymer hybrid carrier for cell internalization. React Funct Polym 124: 72-76.
- Gao J, Zhao B, Wang M, Serrano MA, Zhuang J, et al. (2018) Supramolecular assemblies for transporting proteins across an immiscible solvent interface. J Am Chem Soc 140(7): 2421-2425.
- Dutta D, Fauer C, Hickey K, Salifu M, Stabenfeldt SE (2017) Tunable delayed controlled release profile from layered polymeric microparticles. J Mater Chem B 5(23): 4487-4498.
- Guerreiro LH, Silva D da, Girard-Dias W, Mascarenhas CM, Miranda K, et al. (2017) Macromolecular confinement of therapeutic protein in polymeric particles for controlled release: insulin as a case study. Braz J Pharm Sci 53(2): e16039.
- Menon JU, Ravikumar P, Pise A, Gyawali D, Hsia CCW, et al. (2014) Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater 10(6): 2643-2652.
- Yin L, Yuvienco C, Montclare JK (2017) Protein based therapeutic delivery agents: Contemporary developments and challenges. Biomaterials 134: 91-116.
- Gregersen N, Bross P, Vang S, Christensen JH (2006) Protein misfolding and human disease. Annu Rev Genomics Hum Genet 7: 103-124.
- Coyne J, Davis B, Kauffman D, Zhao N, Wang Y (2017) Polymer microneedle mediated local aptamer delivery for blocking the function of vascular endothelial growth factor. ACS Biomater Sci Eng 3(12): 3395- 3403.
- Dhoot N (2002) Microencapsulation for therapeutic applications. Drexel University, USA, pp. 1-237.
- Hendrickson WA, Ward KB (1975) Atomic models for the polypeptide backbones of myohemerythrin and hemerythrin. Biochem Biophys Res Commun 66(4): 1349-1356.
- Chow YW, Pietranico R, Mukerji A (1975) Studies of oxygen binding energy to hemoglobin molecule. Biochem. Biophys Res Commun 66(4): 1424-1431.
- Dai C, Wang B, Zhao H (2005) Microencapsulation peptide and protein drugs delivery system. Colloids Surf B Biointerfaces 41(2-3): 117-120.
- Pisal DS, Kosloski MP, Balu-Iyer SV (2010) Delivery of therapeutic proteins. J Pharm Sci 99(6): 2557-2575.
- Horber FF, Haymond MW (1990) Human growth hormone prevents the protein catabolic side effects of prednisone in humans. J Clin Invest 86(1): 265-272.
- Schäcke H, Döcke WD, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96(1): 23-43.
- Lewis G, Jusko W, Burke C, Graves L (1971) Prednisone side-effects and serum-protein levels: A Collaborative Study. The Lancet 2(7728): 778- 780.
- Bispo LB (2015) Recombinant protein for bone augmentation in implantology. Rev Bras Odontol 72(1-2): 30-36.
- Santos AAD, Miranda CDO, Alves MTS, Faloppa F (2005) O papel da proteína morfogenética óssea na reparação do tecido ósseo. Acta Ortopédica Bras 13(4): 194-195.
- Spector DI, Keating JH, Boudrieau RJ (2007) Immediate mandibular reconstruction of a 5cm defect using rhBMP-2 after partial mandibulectomy in a dog. Vet Surg 36(8): 752-759.
- Oliveira ORG, Martins SPR, Lima WG, Gomes MM (2017) The use of bone morphogenetic proteins (BMP) and pseudarthrosis, a literature review. Rev Bras Ortop Engl 52(2): 124-140.
- Richard NW, Green J, Wang Z, Deng Y, Qiao M, et al. (2014) Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes & Diseases 1(1): 87-105.
- Coskuner O, Uversky VN (2017) BMP-2 and BMP-9 binding specificities with ALK-3 in aqueous solution with dynamics. J Mol Graph Model 77: 181-188.
- Li X, Yi W, Jin A, Duan Y, Min S (2015) Effects of sequentially released BMP-2 and BMP-7 from PELA microcapsule-based scaffolds on the bone regeneration. Am J Transl Res 7(8): 1417-1428. 42. Reguera-Nuñez E, Roca C, Hardy E, de la Fuente
- Reguera-Nuñez E, Roca C, Hardy E, de la Fuente M, Csaba N, et al. (2014) Implantable controlled release devices for BMP-7 delivery and suppression of glioblastoma initiating cells. Biomaterials 35(9): 2859- 2867.
- Rajesh G, Paul A, Mishra SR, Bharati J, Thakur N, et al. (2017) Expression and functional role of Bone morphogenetic proteins (BMPs) in cyclical corpus luteum in buffalo (Bubalus bubalis). Gen Comp Endocrinol 240: 198-213.
- Wang C, Lu WW, Wang M (2017) Bicomponent fibrous scaffolds made through dual-source dual-power electrospinning: Dual delivery of rhBMP-2 and Ca-P nanoparticles and enhanced biological performances. J Biomed Mater Res A 105(8): 2199-2209.
- Egashira K, Sumita Y, Zhong W (2018) Bone marrow concentrate promotes bone regeneration with a suboptimal-dose of rhBMP-2. PloS One 13(1): e0191099.
- Y, Wang M, Liu W, Chen C, Cui W, et al. (2017) Chitosan/nHAC/PLGA microsphere vehicle for sustained release of rhBMP-2 and its derived synthetic oligopeptide for bone regeneration. J Biomed Mater Res A 105(6): 1593-1606.
- Zheng GB, Yoon BH, Lee JH (2017) Comparison of the osteogenesis and fusion rates between activin A/BMP-2 chimera (AB204) and rhBMP-2 in a beagle’s posterolateral lumbar spine model. Spine J 17(10): 1529- 1536.
- Baldus C, Kelly MP, Yanik EL, Drake BF, Ahmad A, et al. (2017) Incidence of cancer in spinal deformity patients receiving high-dose (≥40 mg) bone morphogenetic protein (rhBMP-2). Spine 42: 1785-1791.
- Yu NY, Fathi A, Murphy CM, Mikulec K, Peacock L, et al. (2017) Local co-delivery of rhBMP-2 and cathepsin K inhibitor L006235 in poly (d, l-lactide-co-glycolide) nanospheres. J Biomed Mater Res B Appl Biomater 105(1): 136-144.
- Quaas B, Burmeister L, Li Z, Nimtz M, Hoffmann A, et al. (2018) Properties of dimeric, disulfide-linked rhBMP-2 recovered from E. coli derived inclusion bodies by mild extraction or chaotropic solubilization and subsequent refolding. Process Biochem 67: 80-87.
- Chatzinikolaidou M, Pontikoglou C, Terzaki K, Kaliva M, Kalyva A, et al. (2017) Recombinant human bone morphogenetic protein 2 (rhBMP-2) immobilized on laser-fabricated 3D scaffolds enhance osteogenesis. Colloids Surf B Biointerfaces 149: 233-242.
- Chai Y, Lin D, Ma Y, Yuan Y, Liu C (2017) RhBMP-2 loaded MBG/ PEGylated poly (glycerol sebacate) composite scaffolds for rapid bone regeneration. J Mater Chem B 5(24): 4633-4647.
- Zhang M, Ma Y, Li R, Zeng J, Li Z, et al. (2017) RhBMP-2-loaded Poly (lacticco-glycolic acid) microspheres fabricated by coaxial electrospraying for protein delivery. J Biomater Sci Polym 28(18): 2205-2219.
- Gohil SV, Wang L, Rowe DW, Nair LS (2018) Spatially controlled rhBMP-2 mediated calvarial bone formation in a transgenic mouse model. Int J Biol Macromol 106: 1159-1165.
- Parry JA, Olthof MG, Shogren KL, Dadsetan M, Van Wijnen A, et al. (2017) Three-dimension-printed porous poly (propylene fumarate) scaffolds with delayed rhBMP-2 release for anterior cruciate ligament graft fixation. Tissue Eng Part A 23(7-8): 359-365.
- Silva HCL, Junior C, Peixoto A, Moreno R, Miranda SL, et al. (2017) Offlabel use of rhBMP-2 as bone regeneration strategies in mandibular ameloblastoma unicystic. Einstein São Paulo 15(1): 92-95.
- Tannoury CA, An HS (2014) Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 14(3): 552- 559.
- Grasiele MM, Rosa S (2013) A tecnologia da microencapsulação através das microesferas de quitosana. Rev Iberoam Polímeros 14(5): 206-218.
- Jyothi NVN, Prasanna PM, Sakarkar SN, Prabha KS, Ramaiah PS, et al. (2010) Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul 27(3): 187-197.
- Costa RC, Pereira ED, Silva FM, Oliveira de Jesus EF, Souza FG (2018) Drug micro-carriers based on polymers and their sterilization. Chem Chem Technol.
- Ansary RH, Rahman MM, Mohamad N, Arrif TM, Latif AZA, et al. (2017) Controlled release of lysozyme from double-walled poly (Lactide-Co- Glycolide)(PLGA) microspheres. Polymers 9(10): 485.
- Holcapkova P, Hrabalíková M, Stoplova P, Sedlarik V (2017) Core-shell PLA-PVA porous microparticles as carriers for bacteriocin nisin. J Microencapsul 34(3): 243-249.
- Paulo F, Santos L (2017) Design of experiments for microencapsulation applications: A review. Mater Sci Eng C 77: 1327-1340.
- Mokhtar M, Gosselin P, Lacasse F, Hildgen P (2017) Design of PEGgrafted- PLA nanoparticles as oral permeability enhancer for P-gp substrate drug model Famotidine. J Microencapsul 34(1): 91-103.
- Delmote J, Teruel-Biosca L, Ribelles JLG, Ferrer GG (2017) Emulsion based microencapsulation of proteins in poly (L-lactic acid) films and membranes for the controlled release of drugs. Polym Degrad Stab 146: 24-33.
- Roointan A, Kianpour S, Memari F, Gandomani M, Gheibi Hayat SM, et al. (2018) Poly (lactic-co-glycolic acid): The most ardent and flexible candidate in biomedicine! Int J Polym Mater Polym Biomater, pp. 1-22.
- Odonchimeg M, Kim SC, Shim YK, Lee WK (2018) Preparation of “Open/ closed” pores of PLGA-microsphere for controlled release of protein drug. Mong J Chem 18: 41-47.
- Batista P, Castro PM, Madureira AR, Sarmento B, Pintado M (2018) Recent insights in the use of nanocarriers for the oral delivery of bioactive proteins and peptides. Peptides 101: 112-123.
- Quinlan E, López-Noriega A, Thompson E, Kelly HM, Cryan SA, et al. (2015) Development of collagen-hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J Controlled Release 198: 71-79.
- Qiao C, Zhang K, Sun B, Liu J, Song J, et al. (2015) Sustained release poly (lactic-co-glycolic acid) microspheres of bone morphogenetic protein 2 plasmid/calcium phosphate to promote in vitro bone formation and in vivo ectopic osteogenesis. Am J Transl Res 7(12): 2561-2572.
- Lochmann A, Nitzsche H, von Einem S, Schwarz E, Mäder K (2010) The influence of covalently linked and free polyethylene glycol on the structural and release proerties of rhBMP-2 loaded microspheres. J Control Release Off J Control Release Soc 147(1): 92-100.
© 2018 Zhenzhen Yu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






