- Submissions

Full Text
Research & Development in Material Science
Biosynthesis of Eco-Friendly Silver Nano-Particles using Dry Fluted Pumpkin (Telfairia occidentalis) Leave Extract as Reducing Agent
Ihom P Aondona*
Department of Mechanical and Aerospace Engineering, University of Uyo, Nigeria
*Corresponding author: Ihom P Aondona, Department of Mechanical and Aerospace Engineering, University of Uyo, PMB 1017 Uyo, Akwa Ibom State, Nigeria
Submission: March 13, 2018;Published: March 28, 2018

ISSN: 2576-8840
Volume5 Issue1
Abstract
The biosynthesis of eco-friendly silver nano-particles using dry fluted pumpkin (Telfairia ocidentalis) leave extract as reducing agent was carried out. The research used the extract which was prepared from dry fluted pumpkin leaves to reduce 0.01M silver nitrate solution without the addition of any capping agent. During the biosynthesis process qualitative measures like 15 minutes interval observation for 1hr., observation of precipitates formation, and laser light pointer were taken to establish the formation of AgNPs in the solution. The AgNPs for characterization were produced by allowing the biosynthesis solution to remain in the cardboard for 24 hours before filtering and drying the residue in the oven at 110 °C. The residue which was the AgNPs was characterized using ED-XRF and SEM. The NPs produced were of average size of 30nm and angular and cubicle in shape. The chemical composition of the NPs was 71.8% Ag2O, 25% SiO2, 2.18% Cl and other trace compounds. The NPs; given the eco-friendly method of their biosynthesis can be further purified to be used on wound bandage.
Keywords: Reducing agent; Telfairia occidentalis; Nano-particles; Wound; Purity; Extract
Introduction
Manigandan et al. [1] posited that the unique physicochemical properties of nanomaterials are attractive for use in a variety of technologies due to the factors such as conductivity, magnetic property, and optical sensitivity by the characteristics such as small size, shape, surface structure and chemical composition. Modifying the properties of nanoscale materials generally involves control over the physicochemical features of the material. Noble metal nanostructures have created attention due to their extensive applicability in various domains. The advent of nanomaterials and their production sharpened man’s precision in production especially the concept of top-down technique, from bulk to small and even smaller dimensions up to manufacturing molecule-tomolecule or even atom-to-atom and the bottom-up technique [2-4]. There has been resurgence in the manufacture of nano-silver not only as Silver (Ag) is a soft, translucent gray transition metal with an atomic number of 47. This element has the highest thermal and electrical conductivity of all the metallic elements found so far on Earth. The electronic configuration of Silver is [Kr]4d105s1. It can be found in pure form or alloyed with other elements. It is considered a precious metal and is used for coins and bullions as part of monetary systems; it can be found in jewellery and ornaments. The photographic film industry has relied on the photochemical reactivity of silver and silver halide nanoclusters for many years to generate photographs [5].
Because of its close association with human history in several applications, silver has also been tested and used in dentistry in the form of amalgams with mercury for fillings to repair tooth decay. In addition, silver compounds have long been known for their antimicrobial activity. With the advent of a plentiful supply of antibiotics after World War II, there has been a major shift away from metal ions and their antimicrobial effects (oligodynamic effects) until recently. As microbes can evolve, resistant bacterial strains, for example, are capable of handling the latest most potent generations of antibiotics, and coupled with their ability to pass on this resistance to others. It makes them formidable pathogens. Thuantibacterial, but also for potential application against fungi, viruses, and other pathogenic species. There is currently a drive to make nano-silver and apply it as coating for various medical components to stop infections. For instance silver nanoparticles sprayed bandages can be used to tie wounds to avoid bacterial infections. Scientist Robert Burrell created a process to manufacture antibacterial bandages using nanoparticles of silver. Silver ions block microbes cellular respiration. In other words, silver smothers harmful cells killing them [6-10].
Here comes the issue of method of preparation; ecofriendly and harmless substances as reducing agents and capping chemicals are preferred. Any method therefore that will promote safer and purer form of silver nanoparticles for medical application will be a better option [11-13]. In this work the author intends to use fluted pumpkin (Telfairia occidentalis) as both reducing agent and capping material for the production of pure AgNPs that can be applied as coating for various medical components to stop infections; including the treatment of wounds. No wonder the objective of the work which is the biosynthesis of AgNPs using eco-friendly approach of reducing with plant extract.
Materials and Methods
Materials
The materials used for this work were; Telfairia occidentalis (Fluted Pumpkin), the extracts from the leaves were used as reducing agent, and 0.01M solution silver nitrate (AgNO3) was used as the source of silver ions. Also used was milli-Q- water. The leaves used can be seen in Figure 1 below:
Figure 1: Telfairia occidentalis (Fluted pumpkin).

Equipment
The following equipment were used for the research work; 50ml measuring cylinder, 250ml beaker, 250ml conical flask, filter paper, 20ml micropipette, micropipette tip, mortar and pestle, laser pointer. Digital camera, spatula, magnetic stirrer, hot plate (heater), 250ml reagent bottles, digital weighing balance; energy dispersive x-ray fluorescence (ED-XRF), Scanning Electron Microscope (SEM), blender, kimwipes, Buchner funnel, 50ml glass vials, and oven.
Method
Dried leaves extract preparation: Here the procedure varied slight from that of preparing extracts from fresh leaves. Fresh leaves of fluted pumpkin were collected from the University of Uyo, Biological Garden, the leaves were sun-dried after thoroughly washing them. They were again dried in the oven at 40 °C for 24hrs. The leaves were then blended using a blender. From the driedprocessed leaves, 5g was measured and transferred into 250ml beaker to which was poured 50ml milli -Q- water and boiled on the heating plate. The suspension was allowed to cool before it was poured into the funnel with filter paper to filter out the suspension. The extract was collected as filtrate in the beaker Figure 2 below:
Figure 2: Extract from fluted pumpkin (Telfairia occidentalis) dried leaves.

Bio-synthesis of AgNPs using extract of the dried leaves: 180mL of the dried leave extract of Telfari occidentalis was poured into 250ml reagent bottle. Then 20mL of 0.01M AgNO3 was poured into the reagent bottle containing 180ml of the dried leave extract of Telfairia occidentalis to synthesize the Ag NPs. After that, the mixture of the leave extract and AgNO3 was gently shaken for 2min to have a uniform solution of the mixture. After shaking, the mixture was kept still and observed for any colour change after interval of 15min for 60min. Laser beam from a laser pointer was used to observe if there was scattering of the light on the mixture as the beam of light passes through the bottle containing the AgNO3 solution to the bottle containing the mixture of the extract and the AgNO3 Solution.. After observation for 60mins the solution was allowed to stay for 24hrs resulting in more particles being formed; noticed through change of colour and quantity of residue on the filter paper. Figure 3 captures the biosynthesis process of AgNPs using dried leaves of Telfairia occidentalis extract.
Figure 3:
3a:Telfairia occidentalis dried leaves extract
3b:The laser light is slightly scattered by the mixture of Telfaria occidentalis and 0.01M AgNO3.

The mixture of the synthesized Ag-NPs using the extracts from the dried leaves of Telfairia occidentalis was put in a dark cupboard for 24hrs and was later filtered using filter paper. The residue that was deposited on the filter paper was dried in an oven at a temperature of 110 °C for 6 hours to obtain powdered Ag NPs. The process is captured in Figure 4.
Characterisation of AgNPs produced using Telfairia occidentalis (Fluted pumpkin): SEM Analysis was carried out, with Phenom SEM Model Pro x, on the AgNPs produced to identify the morphology, size and shape of the produced AgNPs. The chemical analysis of the AgNPs was performed by using Energy Dispersive X-Ray Flourescent, Mini pal4 ED-XRF model. The two tests were carried out at Nigeria Geological Survey Agency, Zaria, Kaduna.
Figure 4: Silver nanoparticles from dried leaves of Telfairia occidentalis extract without any capping agent.

Results and Discussion
Results
The results of the work are displayed as below; Table 1 gives the result of the AgNPs biosynthesis process, Figure 5 indicates colour at start of biosynthesis and colour change after 1 hour, Table 2 shows some properties during the biosynthesis process, Table 3 shows the chemical analysis of the AgNPs using ED-XRF, and Figure 6 shows the SEM micrographs of the AgNPs.
Figure 5:The colour change of the reaction mixture of silver nitrate and dried leave extract of T. occidentalis
5a:Initial (Light brown)
5b:Colour change (Dark brownish).

Table 1: Biosynthesis of silver nanoparticles using dried Telfairia occidentalis leaves extract.
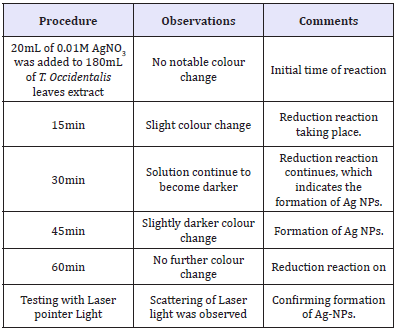
Discussion
Table 1 gives the result of the biosynthesis of AgNPs using dried leaves extract of Telfairia occidentalis (fluted pumpkin). The initial colour of the biosynthesis solution was light brown; this colour continued to change after the interval of 15 minutes up to 1hr, when it was completely changed to dark brown. See Figure 5a & 5b. As the colour change was going on, precipitates were also sighted in the biosynthesis solution indicating that the extract was reducing the Ag+ ions into Ag0 metal [14,15]. The Laser light pointed at the solution revealed scattering in the biosynthesis solution. According to [5], one interesting property of colloidal particles, because of their shape and size, is that they scatter white light in a process called the Tyndall effect. Named after the nineteenth, century physicist john tyndall, the effect is the process of light being scattered and reflected by colloidal particles or NPs in suspension. The presence of a colloidal suspension can be easily detected by the scattering/ reflection of a laser beam from the NPs as the beam of light passes through the solution. In contrast, when the laser beam is shined through a normal solution (i.e. silver nitrate solution) without colloids or NPs, the beam passes through without scattering. The Tyndall effect can only be used to determine if there are colloids/ NPs in that solution. Thus, it acts as a qualitative tool in the rapid determination of AgNPs in this instance because the human eye cannot directly see individual NPs in the solution. The method used in determining the formation of AgNPs agrees with above.
Table 2: Electronic conductivity, ph and temperature during the biosynthesis process.
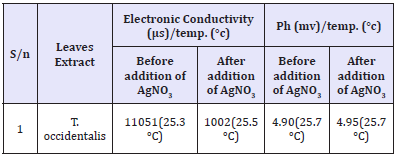
Table 2 gives the electronic conductivity, pH, and temperature change before and during the biosynthesis of the AgNPs using Telfairia occidentalis as the reducing agent. The electronic conductivity was 11051μS and this dropped to 1002μS in the biosynthesis solution; indicating that reduction of Ag+ ions to Ag0 was ongoing in the solution. The pH of the reducing agent which was 4.9 when added to the AgNO3 solution; the pH of the biosynthesis solution became 4.95; reducing in acidity. The temperature change was not significant during the biosynthesis process. The potentials of leave extracts as a reducing agent for biosynthesis of AgNPs has been confirmed by several authors. In some plants, the acidic components can easily aid the reduction of the metallic ions. Furthermore, these studies showed that AgNPs created this way possesses good antimicrobial activity. The fact that no capping agent or templating agent is needed makes this chemical route an attractive one. No capping agent was used in this work except the Telfairia occidentalis extract which served as both reducing and capping agent.
Table 3: Result of ED-XRF analysis.
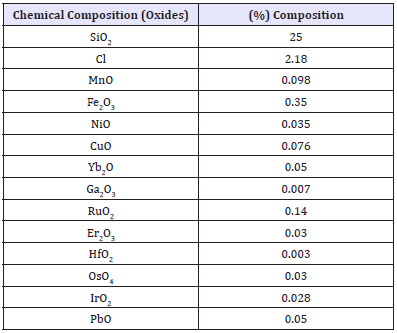
Figure 6 : AgNPs size and shape at different magnifications.

The biosynthesis solution was hid in the cardboard for 24 hours before it was removed and filtered. The residue on the filter paper was dried in the oven at 110 °C to produce dry AgNPs before it was sent for characterization. See Table 3 for the chemical composition of the AgNPs and Figure 6 for the morphology, size and shape of the silver nanoparticles which were produced using Telfairia occidentalis as a reducing agent for silver nitrate solution. The optical property of silver NPs is tunable through the visible and near-infrared region of the spectrum as a function of NP size, shape, aggregation state, and local environment. The size and shape of the Silver NPs and the viewing conditions (transmitted light or reflected light) determine the colour of the silver solution that is ultimately obtained. The average size of the NPs were 30nm and they were mostly angular and cubicle in shape. The chemical composition showed that Ag2O was 71.8%, SiO2 was 25% and Cl was 2.18%, the other compounds were trace compounds. The instrument used for the analysis was calibrated to measure oxides and may have picked the oxygen from the surface of the NPs and not from the core of the NPs. The purity of the NPs can further be enhanced if for instance it is to be used for wound bandages.
Conclusion
This work has examined and studied the research work ‘’Biosynthesis of eco-friendly silver nano-particles using dried fluted pumpkin (Telfairia occidentalis) leaves extract as reducing agent’’ the details of the conclusions drawn from this study are here presented:
i. The work has shown that dried fluted pumpkin leave extract can be used as a reducing agent without any capping to produce eco-friendly AgNPs
ii. Laser light pointer, colour change and precipitates formation was qualitative proofs that the dried fluted pumpkin leave extract reduced the Ag+ ions to AgNPs
iii. The chemical composition of the AgNPs showed that it contained 71.8% Ag2O; the purity of which can be increased by removing the 25% SiO2, 2.18% Cl and other trace elements
iv. The AgNPs produced were of the average size of 30nm
v. The AgNPs produced were angular and cubicle in shape
vi. Given the friendly manner in which the particles were synthesized with further washing and purification they can be used on wound bandage.
Acknowledgment
The researchers of this work who are also the authors of this work wish to sincerely thank all who contributed in one way or the other in making this research work a reality. Our acknowledgement goes to the following: Engr. Peter Asangasung, of the Chemical Engineering Laboratory, University of Uyo-Nigeria and Analyst: E.D. Yaharo, Principal Lab. Superintendent I at NSRMEA Kaduna. Finally, we thank Engr. Nicholas Agbo of the Defence Industries Corporation of Nigeria.
References
- Manigandan R, Praveen KS, Munusamy S, Dhanasekarau T, Padnanabau A, et al. (2017) Green biosynthesis of silver nanoparticles using aqueous urginea indica bulbs extract and their catalytic activity towards 4-NP. MMS&E Journal 10: 1-6.
- Mikhail KI (2007) Graphene: carbon in two dimensions. Materials Today 10(1-2): 20-27.
- Preetha NK, Rani J (2012) Nanokaolin clay as reinforcing filler in nitrile rubber. International Journal of Scientific & Engineering Research 3(3): 1-5.
- Obikwelu DON (2012) Nanoscience and nanotechnology-an introduction, (1st edn), De-adroit innovation Enugu, Nigeria.
- Poinern GEJ (2015) A Laboratory course in nanoscience and nanotechnology, (1st edn), CRC Press, Taylor & Francis Group, London, England, pp. 95-101.
- Kuldeep P, Pooja K, Rajesh P (2012) Recent advances in nanotechnology. International Journal of Scientific & Engineering Research 3(11): 1-4.
- Biswas PK, Dey S (2015) Research article effects and applications of silver nanoparticles in different fields. International Journal of Recent Scientific Research 6(8): 5880-5883.
- Shreya M, Amita H, Uttiya D, Paulomi B, Naba KM (2015) Biosynthesis of silver nanoparticles from aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp., Springer. Applied Nanoscience 5(7): 875-880.
- Benakashani F, Allafchian AR, Sah J (2016) Biosynthesis of silver nanoparticles using Capparis spinosa l. leaf extract and their antibacterial activity. Karbala International Journal of Modern Science 2(4): 251-258.
- Bansal P, Duhan JS, Gahlawat SK (2017) Biosynthesis of nanoparticles: A review. African Journal of Biotechnology 13(28): 2778-2785.
- Leela A, Vivekanandan M (2008) Tapping the unexploited plant resources for the synthesis of silver nanoparticles. African Journal of Biotechnology 7(17): 3162-3165.
- Jannathul MF, Lalitha P (2015) Biosynthesis of silver nanoparticle and its application. Journal of Nanotechnology pp. 1-18.
- Selvam K, Sadhakur C, Goverthanan M, Periasamy T, Arumugam S, et al. (2017) Eco-friendly biosynthesis and characterization of silver nanoparticles using tinospora cordfolia (thunb) miers and evaluate its antibacterial, antioxidant potential. Journal of Radiation Research and Applied Sciences 10(1): 6-12.
- (2012) What is nanotechnology? Definition. Nano Work.
- Elnashaie SS, Danafar F, Hashemipour H (2015) Nanotechnology for chemical engineers. Springer Science Plus Business Media, Singapore.
© 2018 Ihom P Aondona. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






