- Submissions

Full Text
Research & Development in Material Science
Characterization of Nariginase Obtained from Aspergillus niger by Submerged Fermentation Using Naringin Extracted from Lemon Peels
Igbonekwu A, Omeje KO, Ezugwu AL, Eze SOO*, Njoku OU and Chilaka FC
Department of Biochemistry, University of Nigeria, Nigeria
*Corresponding author: Eze SOO, Department of Biochemistry, University of Nigeria, Enugu State, Nigeria
Submission: January 09, 2018;Published: March 27, 2018

ISSN: 2576-8840
Volume4 Issue5
Abstract
Naringin was extracted from lemon peels with a yield of 0.25%. The activity of crude naringinase was 157.7μmol/min and 493.64μmol/ min using naringin disappearance and reducing sugars appearance assay methods respectively. Naringinase was precipitated at 50% ammonium sulphate saturation. After gel filtration, a purification fold of 32.84 with specific activity of 7279.37μmol/min/mg was observed while using naringin disappearance assay method and a 20.48 purification fold with specific activity of 14463.57μmol/min/mg with reducing sugars appearance method of assay. Percentage yields of 11.63% and 7.38% were observed using naringin disappearance and reducing sugars appearance methods of assay respectively. The enzyme pH and temperature optima were found to be 3.50 and 50 °C with a Km and Vmax obtained as 5.8mg/ml and 1111.11μmol/ min respectively. This investigation suggests that lemon peels could be a good source of naringin which could be used as a carbon source in submerged fermentation for naringinase production using Aspergillus niger.
Keywords: Naringin; Naringinase; Aspergillus niger; Submerged fermentation and lemon peels
Introduction
Bitterness is the major limiting factor for commercial acceptance of processed citrus fruit such as juice, wine, and vinegar [1]. The bitterness in citrus fruit is affected by limonin and naringin which are flavonoids generally recognized for making juice bitter [2]. Naringin which was first found in grapefruit is abundant in immature fruit with its concentration decreasing as fruit ripens [3]. Naringin has sugar complexes (α-L-rhamnose and β-D-glucose) and an aglycone (Naringenin) part. Naringinase is a bienzyme consisting of a-L-rhamnosidase (EC 3. 2.1.40) and flavonoid-bglycosidase (EC 3.2.1.21). b-L-Rhamnosidase hydrolyses naringin into the flavonoid prunin, which is converted to naringenin (4,5,7- trihydroxyflavonone) by b-D-glucosidase. It has been observed that Naringinase catalyzes the hydrolysis of naringin to naringenin, glucose, and rhamnose. Fruit Juice industries process fruits at the lowest possible cost while maintaining the organoleptic quality and stability of the finished products, with increased consumer acceptability [4].
Numerous techniques used in naringin reduction including adsorptive debittering and chemical methods have limitations by altering nutrient composition by either chemical reactions or removal of nutrients, flavor and color [3]. A suitable debittering is achieved by treating the juice with naringinase. The enzyme naringinase hydrolyses naringin and produces a tasteless compound naringenin by two step reactions [5]. The reduction in bitterness as a result of the enzymatic process controls the quality and increase the acceptance by the consumers. Therefore, debittering with naringinase will not reduce the health promoting effects of fruit juices [6]. Naringinase has been produced from plants [7-9]. Ilamathi et al. [10] observed that the debittering process could be more cost effective and economically viable if naringinase production is achieved industrially using microorganisms.
In recent times, naringinase has been produced from several bacteria such as; Penicillium decumbens [11], Candida tropicalis [12], Aspergillus niger [13,14], Aspergillus Oryzae JMU 316 [1], and Penicillium purpurogenum [5], using different methods. Up till now there is a dearth of information on production of nariginase from Aspergillus niger using submerged fermentation. Kanokpan et al. [13] have only carried out preliminary enzyme characterization and optimization of culture parameters for production of Naringinase isolated from Aspergillius niger. This work is aimed at extraction of naringin and production of naringinase from Aspergillus niger in submerged fermentation system using the extracted naringin as the only carbon source.
Materials and Methods
Collection of lemon fruits: Mature lemon fruits (Citrus limon) were bought from New market, Enugu, Enugu State, Nigeria.
Collection of fungi culture: Aspergillus niger was a generous gift from Postgraduate Laboratory, Department of Microbiology, University of Nigeria.
MethodsExtraction of naringin: Naringin was extracted from the lemon peels using the method of [15].
Protein determination: Protein was determined by the method of Lowry et al. [16] with bovine serum albumin (BSA) as a standard.
Production of naringinase: Naringinase was produced using the method of Kanokpan et al. [13].
Assay of Naringinase Activity
Naringinase activity was assayed in two ways:
By monitoring the magnitude of naringin disappearance
Naringinase activity was assayed using the method described by Ribeiro & Rabaca [5] with the following modifications. 0.3ml 0.1M sodium acetate buffer pH 4.0 was added to 1ml of 5% naringin. Thereafter, 0.2ml of the enzyme was added. This was incubated at 50 °C for 1hr. 0.1ml of the incubated reaction mixture was added to 5ml of 90% diethylene glycol containing 0.1ml of 4M NaOH and allowed to stand for 10min at room temperature. The residual naringin was then measured at 420nm using a JENWAY 6405 UV/VIS spectrophotometer (Beckman/Instruments, Inc., Huston Texas). One unit (U) of naringinase activity is defined as the amount of enzyme that hydrolyzes 1μmol of naringin per minute under the assay conditions.
By monitoring the appearance of reducing sugar
The reducing sugars were quantified by the 2, 4-dinitrosalicyclic acid (DNS) method of Millers (1959) with the following modifications; 0.3ml of sodium acetate buffer (0.1M pH 4.0) was added to 1ml of 5% naringin followed by the addition of 0.2ml of the enzyme. This was allowed to stand for 1hr at 50 °C. 1ml of DNS was added. The mixture was boiled for 10min using a water bath. 1ml of sodium potassium tartarate was added and the mixture was brought to 0 °C under ice blocks. The absorbance was then read at 540nm using a JENWAY 6405 UV/VIS spectrophotometer (Beckman/Instruments, Inc., Huston Texas). One unit of naringinase activity is defined as the amount of enzyme that catalyzes the release of 1μmole of glucose and rhamnose per minute under the assay conditions.
Effect of incubation time on naringinase activity
Different test tubes (pyrex) containing 1ml of substrate, 0.3ml of sodium acetate buffer (0.1M pH 4.0) and 0.2ml crude enzyme were incubated at 50 °C for varying period of time (min) ranging from 0-120. At the end of each incubation period, the reaction mixture was assayed for naringinase activity as earlier described.
Enzyme purification
Partial purification of the naringinase: purified by a modification of the method of Chen et al. [17]. The crude enzyme was brought to 50% (NH4)2SO4 saturation with solid (NH4)2SO4. This was kept at 4 °C for 30hr, thereafter it was centrifuged at 4000rmp for 30min using a Cole-palmer VS-13000 micro centrifuge. The precipitate was collected and redisolved in 0.1M acetate buffer pH 4.0. 10ml of the redissolved protein was introduced onto a sephadex G-200 gel chromatographic column (50x2.5cm) for gel filtration. The column was pre equilibrated with 0.1M sodium acetate buffer pH 4.0. Fractions were collected at a flow rate of 5ml/9min. The protein concentration of the eluents was monitored at 280nm using a JENWAY 6405 UV/VIS spectrophotometer (Beckman/Instruments, Inc., Huston Texas). The naringinase activity of the collected fractions was assayed as earlier described and the active fractions were pooled and stored at -10 °C.
Effect of pH on naringinase activity: Naringinase activity was determined by a modification of the method of Ghada et al. [18]. In the following assay buffer systems using the assay methods earlier described; 0.1M sodium acetate buffer pH 3.5-5.5, 0.1M sodium phosphate buffer pH 6.0-7.5 and 0.1M Tris-HCl buffer 8.0-9.0.
Effect of temperature on naringinase activity: Naringinase activity was determined at various temperatures; 30, 40, 50, 60, 70 and 80 °C using a modification of the method of Ghada et al. [18]. The lag phase was minimized by preincubating the reaction cocktail in a circulating water bath at the desired temperature for 10min followed by the introduction of 0.1ml of the enzyme solution which was rapidly mixed and assayed as earlier described.
Effect of Substrate Concentration on naringinase activity: The kinetics of Naringinase reaction (Km and Vmax) was determined in duplicate by a modification of the method of Ribeira & Rabaca [19]. The enzyme was incubated with 5, 10, 15, 20, 25 and 30mg/ ml of lemon naringin at pH 3.5 and 50 °C.
Results and Discussion
Figure 1: Monitoring the day of highest protein production from Aspergillus niger grown on naringin from lemon peel.
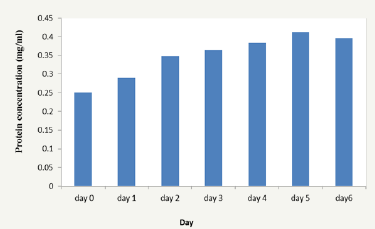
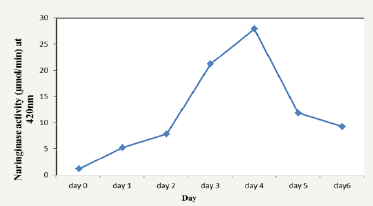
Naringin was extracted from lemon peels with a yield of 0.25%. Naringinase was produced by A. niger in a submerged fermentation system using naringin as the only carbon source. Pilot study was carried out to monitor the day of highest naringinase activity and production. The highest naringinase concentration was obtained on day five while the highest naringinase activity was obtained on day four of fermentation (Figure 1 & 2). Different periods of fermentation (days) have been reported for optimum naringinase activity. Chen et al. [20] reported day five while Radhakrishnan et al. [21] observed highest activity on day seven. Saranya et al. [12] obtained maximum activity on day five.
Figure 2: Determination of the day of highest naringinase activity (μmol/min).
The effect of period of incubation on the activity of naringinase using naringin as the only carbon source was investigated. 55min of incubation time yielded the highest Naringinase activity of 27039.84μmol using the reducing sugar production method of assay (Figure 3).
Crude naringinase was partially purified on a two-step purification step of ammonium sulphate precipitation and gel filtration. 50% ammonium sulphate saturation yielded naringinase with a specific activity of 335.40U/mg using naringin hydrolysis method of assay and 846.60U/mg using reducing sugars production assay method (Table 1 & 2). Saranya et al. [12] reported a specific activity of 0.061U/mg using naringin hydrolysis assay method upon 65% ammonium sulphate precipitation. Figure 4 & 5 show the elution profiles of naringinase on gel filtration with sephadex G-200 using the two methods of assay. A 32.84 and 20.48 purification folds were observed with specific activities of 7279.37μmol/min/ mg and 14463.57μmol/min/mg upon gel filtration using naringin disappearance assay method and reducing sugars appearance assay methods respectively (Table 1 & 2). The percentage yields were found to be 11.63% using naringin disappearance assay method and 7.38% using reducing sugars production assay methods. Saranya et al. [12] reported specific activity of 1.307U/ mg using reducing sugar production assay method which is very low compared to the one obtained in this work.
Figure 3: Progress curve of naringinase activity by studying the production of glucose and rhamnose.
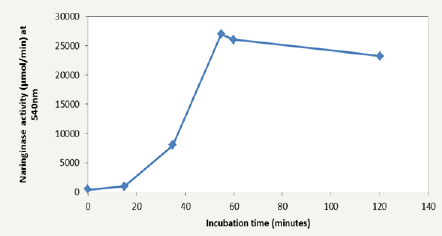
Table 1: Purification table for naringinase by studying naringin hydrolysis.

Table 2: Summary of purification steps for naringinase by studying reducing sugar production.

Figure 4: Elution profile on gel chromatography using sephadex G200.
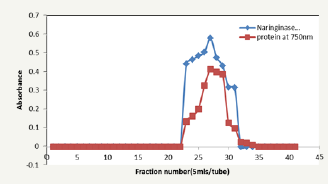
Figure 5: Elution profile on gel chromatography using sephadex G200.
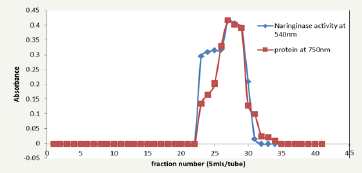
Figure 6: Effect of on naringinase activity on naringin hydrolysis.
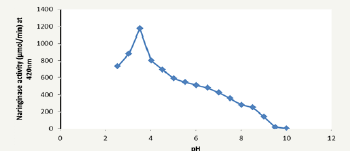
Figure 6 shows an optimum pH of 3.5. Nourouzian et al. [22] reported a pH optimum of 4.0 using Pencillium decumbens while Puri et al. [23] reported a pH of 4.0 using Aspergillus niger. The observed pH was comparatively lower than the report of the above authors but comparatively higher than 3.0 reported by Saranya et al. [12] using Candida tropicalis. In Figure 7, as temperature increased from 25 °C to 50 °C, the naringinase activity increased. Further, increase in temperature beyond of 50 °C decreased naringinase activity making 50 °C the optimum temperature. The decrease in naringinase activity at higher temperature may be due to enzyme denaturation or inactivation. Hence the optimum temperature 50 °C was used for further studies. The optimum temperature is comparatively high when compared to 25 °C obtained using Candida tropicalis but lower when compared to 55 °C obtained using Penicillium decumbens and higher compared to 45 °C obtained using Aspergillus niger [21-23].
Figure 7: Effect of temperature on naringinase activity during hydrolysis and appearance of reducing sugar.
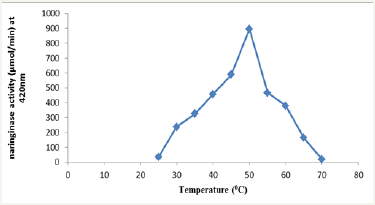
Figure 8: Lineweaver-Burk plot of initial velocity data at different naringin concentration.
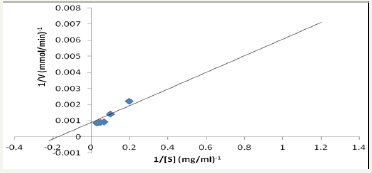
The Km and Vmax obtained from Linewaever-Burk plot of initial velocity data at different concentrations of substrates (Figure 8) were found to be 7.1mM and 1000μmol/min. This shows that partially purified naringinase obtained in this work has high affinity for naringin from grape peel and could be of industrial use. Saranya et al. [12] reported Km and Vmax of 0.19mM and 0.1μmole/ min, using Candida tropicalis which were lower than the Km and Vmax obtained in this work. Also, Nourouzian et al. [22] reported Km of 1.7mM using Penicillum decumbens.
Conclusion
The results obtained in this work have shown that lemon peels could be a good source of naringin which could be used as a carbon source in submerged fermentation for naringinase production using Aspergillus niger. This is also a promising way of converting waste to wealth.
Acknowledgment
The authors wish to thank Eze Francisca O of Dean’s office faculty of Biological Sciences of the University of Nigeria for typing the manuscript.
References
- Xioo D, Tian CG, Hui NC (2010) Optimizing culture, medium for debittering constitutive enzymenarignase production by Aspergillus oryzae JMU 3/6. African Journal of Biotechnology 9(31): 4970-4978.
- Iness JK, Ibrahim M (2013) Characterization of bioactive compounds in Tunisian bitter orange (Citrus auvantium L.) peel and juice and determination of their antioxidant activities. Bio Med Res Int 2013: 345415.
- Puri M, Banerjee UC (2000) Production, purification and characterization of the debittering enzyme naringinase. Biotechnol Adv 18(3): 207- 217.
- Ferreira L, Afonso C, Real VH, Alfaia A, Ribeiro MHL (2008) Debittering of grapefruit juice with naringinase. Food Technology and Biotechnology 46(2): 146-150.
- Ribeiro MHL, Rabaca M (2011) Cross-linked enzyme aggregates of naringnase: novel biocatalysts for naringin hydrolysis. Enzyme Res 2011: 851272.
- Mutuswamy S, Vaidyanathan VK, Manickvassham H, Vargese M, Pothiyappa K (2011) Solid state fermentation for the production of debittering enzyme naringnase using Aspergillus niger MTCC1344. Engineering in Life Sciences 11(3): 322-325.
- Param P, Navjot K (2015) Characterization of enzymes naringnase and the production of debittered low alcoholic Kinnow (Citrus raticulata blanco) beverage. International Journal of Advanced Research 3(6): 1220-1233.
- Cai MA, Glory CL, Uc LG, Vazquez OE (2010) Naringnase production from filamentous fungi using grapefruit rind in solid state fermentation. African Journal of Biotechnology 4(19): 1964-1969.
- Abeer NS, Abeer AEA (2014) Optimization of process parameters by statistical experimental designs for the production of Naringnase enzyme by marine fungi. International Journal of Chemical Engineering 273533: 1-10.
- Ilamaltic R, Shanmugam S, Sattish K (2013) Isolation and characterization of enzymes Naringnaase from A. flavus. International Journal of Advanced Biotechnology and Research 4(2): 208-212.
- Nourouzian D, Osseinzadeh A, NouriInanlou D, Mozami N (2000) Production and partial purification of naringinase from Pencillium decumbens PTCC 5248. World Journal of Microbiology and Biotechnology 16(5): 471-473.
- Saranya D, Shanmugam, Sathish S, Thayumanavan T, Rajasekaran P (2009) Purification and characterization of naringinase from Candida tropicalis. Advanced Biotechnology 3: 11-13.
- Kanokpan T, Pongsuda P, Vitchuporn J, Patjaraporn W (2008) Isolation, preliminary enzyme characterization and optimization of culture parameters for production of naringinase isolated from Aspergillus niger BCC 25166. Kasetsart Journal of Natural Sciences 42: 61-72.
- Vaidyanathan V, Periasamy K, Subash RB (2010) Optimization of fermentation parameters for enhanced production of Naringinase by soil Isolate Aspergillus niger VB07. Food Science Biotechnology 19(3): 827- 829.
- Crandall PG, Kesterson JW (1976) Recovery of naringin and pectin from grapefruit albedo. Proceeding Florida State Horticulture Society 89: 189-191.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 193: 265-275.
- Chen Y, Hui N, Chen F, Nong HC, Lijun L, et al. (2013) Purification and characterization of a Naringinase from Aspergillus aculetus JMUdb058. Journal of Agric Food Chemistry 61(14): 931-938.
- Ghada EAA, Abeer AA, Abeer NS, Mohamed EH, Magdy ME (2016) Covalent immobilization of microbial naringinase using novel thermally stable biopolymer for hydrolysis of naringin. Biotechnology 6(1): 14-20.
- Ribeiro MHL, Rabaca M (2011) Cross-linked enzyme aggregates of naringnase: novel biocatalysts for naringin hydrolysis. Enzyme Research, p. 051272.
- Chen D, Niu T, Cai H (2010) Optimization of culture medium, for debittering enzyme naringinase production by Aspergillus oryzae. African Journal of Biotechnology 9(31): 4970-4978.
- Radhakrisnan I, Shanmugam S, Satishkumar T (2012) Optimization of medium composition for improving naringinase activity using response surface methodology. International Journal of Bio-technology and Research 2: 29-36.
- Nourouzian D, Osseinzadeh A, NouriInanlou D, Mozami N (2000) Production and partial purification of naringinase from Pencillium decumbens PTCC 5248. World Journal of Microbiology and Biotechnology 16(5): 471-473.
- Puri M, Banerjee A, Banerjee UC (2005) Optimization of process parameters for the production of naringinase by Aspergillus niger MTCC 1344. Process Biochemistry 40(1): 195-201.
© 2018 Eze SOO. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






