- Submissions

Full Text
Research & Development in Material Science
An Attempt to Study MoO3-Like TCO Nanolayered Compound in Terms of structural and Ethanol Sensitivity Application
N. Benameur1, M.Ghamnia1 and Boukhachem A2*
1Department of Physics, University of Oran, Algeria
2Faculty of Sciences, Tunis University, Tunisia
*Corresponding author: Boukhachem A, Faculty of Sciences, Unit of physics of semiconductor devices, Tunis University, 2092 Tunis, Tunisia
Submission: December 07, 2017; Published: February 23, 2018

ISSN: 2576-8840Volume4 Issue1
Abstract
MoO3 thin films were successfully synthesized by a simple, inexpensive, and reproducible spray technique for gas sensing applications by using Ammonium molybdate tetrahydrat [(NH4)6Mo7024.4H20]10-2M aqueous solution. XRD shows that MoO3 prepared thin films crystallizes in an orthorhombic structure with space group Pnma (62) related to a-MoO3 phase. On the other hand, AFM observations show some fibers-shape parallel to the substrate. Finally, ethanol detection test shows good sensitivity with reproducible behavior.
Keywords: Molybdenum oxide; Spray; Gas sensors
Introduction
During the last years, the semiconductors based on metal oxides have known many interests in the research as well as in the field of the application. Indeed, these oxides have been applied in several areas such as, optical windows [1], surface acoustic devices [2] and solar cells [3]. In the field of the environment, oxides have played an important role, particularly in photocatalysis and gas detection.
Several metal oxides have been tested as gas sensors such as ZnO [4], In2O3 [5], SnO2 [6].
Recently, MoO3 [7-10] is starting to take a place in the field of the environment.
Thin binary belongs to metallic transition element has emerged as a promising active oxide for various applications. Also, it is found that MoO3 is a n-type semiconductor this property is related to oxygen vacancies in this binary compound. This binary exists in three main crystal structures which are orthorhombic a-MoO3, monoclinic p-MoO3 and hexagonal h-MoO3 [11-14]. Particularly, a-MoO3 has been considered as a potential photocatalyst material [15,16] .
Numerous methods have been carried out to prepare MoO3 thin films, such as MOCVD [17], chemical vapor transport (CVT) [18], sputtering [19] laser ablation [20] and spray pyrolysis [21,22].
The latter is best suited for the preparation of thin films due to its performance in terms of the thin films homogeneity, the possibility of giving crystallized films without resorting to annealing and the possibility of controlling the grains size by controlling the deposition conditions. The present work is devoted essentially to the study of ethanol detection by MoO3 sprayed thin film.
MoO3 Films Preparation and Characterization Techniques
Molybdenum oxide thin films were deposited on glass substrates at 460 °C using 10-2M Ammonium molybdate tetrahydrat [(NH4)6Mo7O24.4H2O] aqueous solution. Spraying was assisted by a stream of nitrogen ensuring good fragmentation of the drops of the solution,. During the deposition process, the precursor mixture flow rate was taken constantly at 4mL/min and the nozzle- to-substrate plane distance was fixed at 27cm.
The formation reaction of molybdenum trioxide from the solution of this precursor can be described by the following reaction:
(NH4) 6Mo7O244H2O → 7MoO3 (s) + 6NH3 (g) + 7H2O(g) (1)
After thin film preparation, a systematic study by XRD was carried out for the determination of films' structure. The atomic force microscope was used for morphological study. On the other hand, the Finally, The sensing properties of MoO3 films based sensors under ethanol vapor at 300°Cwere Investigated.
Results and Discussion
Structural properties
XRD spectrum of prepared thin film (Figure 1) depicts the presence of (020), (040), (131) and (261) peaks related to a-MoO3 orthorhombic phase with (020) and (040 preferred orientations. From thin spectrum, orthorhombic lattice parameters were calculated. Their values are listed in Table 1. These values are closed with others repoirted elsewhere [23,24].
Figure 1:X-ray diffraction spectra of MoO3 thin films.
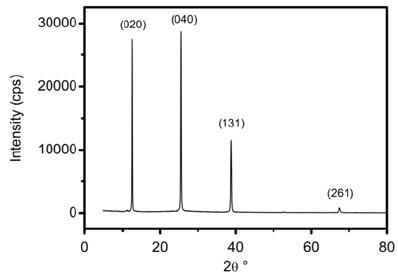
Table 1: Lattice parameters of MoO3 thin films.

Atomic force microscopy (AFM) observations
Surface topography of the MoO3 thin film investigated by atomic force microscopy (AFM) is shown in Figure 2.
Figure 2: 2D AFM topography of MoO3 thin films.
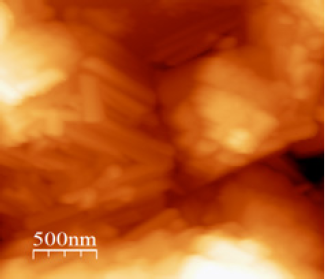
This AFM observation shows that the MoO3 film is formed of parallelepiped shaped wires which can be considered as assembling orthorhombic meshes. The average wires length's is estimated at 500nm and the roughness mean square is around 121nm.
The sensing property of MoO3 films against ethanol vapor was studied. by means of an appropriate experimental protocol. Figure 3 shows MoO3 film responses at 500ppm ethanol measured at 300 °C.
Figure 3: Response of MoO3 sensors to 500ppm ethanol.
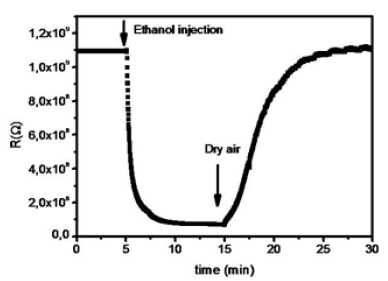
The exploitation of this response allows deducing the sensitivity of the detection which is given by the following relation [25]:
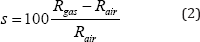
Where Rgas and Rair are the resistance of the device in the presence of ethanol and in air respectively.The value obtained from the sensitivity is estimated at 93%.
This value seams good. Indeed, it is related to a large variation in the resistance value, and therefore a good ethanol sensitivity using molybdenum trioxide. On the other hand, the study of several cycles shows reproducible ethanol detection (Figure 4). This shows that MoO3 can be considered as a potential candidate as ethanol sensors.
Figure 4: Reproducibility Response of MoO3 ethanol sensors to 500ppm ethanol.
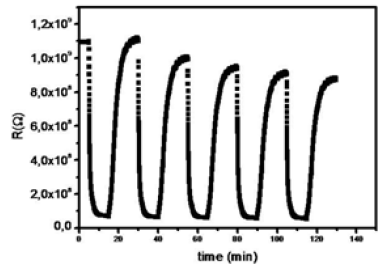
On the other hand, by examining the variation of the resistance of MoO3 sample, it is possible to interpret the mechanism of ethanol sensing as follows.
The reaction mechanism of MoO3 adsorbing ethanol gas is given by:
1/ 2O2 + 2e-→ 2O - (3)
and
CH3CH2OH + 6O-→ 2CO2 +3H2ü + e- (4)
Where O- denotes the oxygen ion on the surface of MoO3 film and e- is a conduction electron. When the zinc oxide film adsorbs ethanol gas, the mobility of electrons in the sensitive film increases because the electrons in the film increase witch results the decreases of MoO3 thin film. The increases of the free carriers can be attributed to the n type MoO3 semiconductor binary compound [26-28]. This can explains the decreases of the resistance following the ethanol adsorption.
Conclusion
The molybdenum trioxide thin films have been successfully prepared on a glass substrate by a simple spray pyrolysis process at 460 °C. X-ray diffraction analysis shows that the prepared film is mainly formed by orthorhombic a-MoO3 phase. The crystallites are preferentially oriented along (020) and (040) directions parallel to the substrate plane.
MoO3 thin films sensors exhibited a high ethanol sensing. This result seems interesting. Indeed, with simple thin film prepared by cost-effective spray pyrolysis technique has been used to prepare such films. Further studies are in progress to improve the sensitivity of MoO3 thin films in terms of effective reproducibility as well as its selectivity.
Acknowledgment
We are very grateful to Professor Ahmed Labidi for his fruitful advices and stimulating discussions.
References
- Han C, Sun Q, Li Z, Dou SX (2016) Thermoelectric enhancement of different kinds of metal chalcogenides. Advanced Energy Materials 6(15): 1600498.
- Grotzinger JP (2013) Analysis of surface materials by the curiosity mars rover. Science 341(6153): 1475-1475.
- Champier D (2017) Thermoelectric generators: A review of applications. Energy Conversion and Management 140: 167-181.
- Zhang L, Wang J, Sun Q, Qin P, Cheng Z, et al. (2017) Three-stage inter- orthorhombic evolution and high thermoelectric performance in Ag-doped nanolaminar snse polycrystals. Advanced Energy Materials 7(19): 1700573.
- Snyder GJ, Toberer ES (2008) Complex thermoelectric materials. Nature materials 7(2): 105-114.
- Pei Y, LaLonde A, Iwanaga S, Snyder GJ (2011) High thermoelectric figure of merit in heavy hole dominated PbTe. Energy & Environmental Science 4(6): 2085-2089.
- Vining CB (2009) An inconvenient truth about thermoelectrics. Nature materials 8(2): 83-85.
- Heremans JP, Dresselhaus MS, Bell LE, Morelli DT (2013) When thermoelectrics reached the nanoscale. Nature nanotechnology 8(7): 471-473.
- Kraemer D, Poudel B, Feng H-P, Caylor JC, Yu B, et al. (2011) Highperformance flat-panel solar thermoelectric generators with high thermal concentration. Nat Mater 10(7): 532-538.
- Leonov V, Vullers RJ (2009) Wearable electronics self-powered by using human body heat: The state of the art and the perspective. Journal of Renewable and Sustainable Energy 1(6): 062701.
- Yang Y, Wei X-J, Liu J (2007) Suitability of a thermoelectric power generator for implantable medical electronic devices. Journal of Physics D: Applied Physics 40(18): 5790-5800.
© 2018 Boukhachem A. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






